Association of Aβ deposition and regional synaptic density in early Alzheimer's disease: a PET imaging study with [11C]UCB-J
- PMID: 33402201
- PMCID: PMC7786921
- DOI: 10.1186/s13195-020-00742-y
Association of Aβ deposition and regional synaptic density in early Alzheimer's disease: a PET imaging study with [11C]UCB-J
Abstract
Background: Attempts to associate amyloid-β (Aβ) pathogenesis with synaptic loss in Alzheimer's disease (AD) have thus far been limited to small numbers of postmortem studies. Aβ plaque burden is not well-correlated with indices of clinical severity or neurodegeneration-at least in the dementia stage-as deposition of Aβ reaches a ceiling. In this study, we examined in vivo the association between fibrillar Aβ deposition and synaptic density in early AD using positron emission tomography (PET). We hypothesized that global Aβ deposition would be more strongly inversely associated with hippocampal synaptic density in participants with amnestic mild cognitive impairment (aMCI; a stage of continued Aβ accumulation) compared to those with dementia (a stage of relative Aβ plateau).
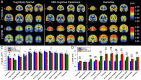
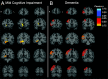
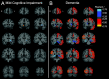
Methods: We measured SV2A binding ([11C]UCB-J) and Aβ deposition ([11C]PiB) in 14 participants with aMCI due to AD and 24 participants with mild AD dementia. Distribution volume ratios (DVR) with a cerebellar reference region were calculated for both tracers to investigate the association between global Aβ deposition and SV2A binding in hippocampus. Exploratory analyses examined correlations between both global and regional Aβ deposition and SV2A binding across a broad range of brain regions using both ROI- and surface-based approaches.
Results: We observed a significant inverse association between global Aβ deposition and hippocampal SV2A binding in participants with aMCI (r = - 0.55, P = 0.04), but not mild dementia (r = 0.05, P = 0.82; difference statistically significant by Fisher z = - 1.80, P = 0.04). Exploratory analyses across other ROIs and whole brain analyses demonstrated no broad or consistent associations between global Aβ deposition and regional SV2A binding in either diagnostic group. ROI-based analyses of the association between regional Aβ deposition and SV2A binding also revealed no consistent pattern but suggested a "paradoxical" positive association between local Aβ deposition and SV2A binding in the hippocampus.
Conclusions: Our findings lend support to a model in which fibrillar Aβ is still accumulating in the early stages of clinical disease but approaching a relative plateau, a point at which Aβ may uncouple from neurodegenerative processes including synaptic loss. Future research should investigate the relationship between Aβ deposition and synaptic loss in larger cohorts beginning preclinically and followed longitudinally in conjunction with other biomarkers.
Keywords: Alzheimer’s disease; Aβ; SV2A; Synaptic density; [11C] PiB PET; [11C]UCB-J PET.
Conflict of interest statement
APM, REC, and CHvD report grants from National Institutes of Health for the conduct of the study. APM reports grants for clinical trials from Genentech and Eisai outside the submitted work. MKC reports research support from the Dana Foundation and research support from Eli Lilly and clinical trials from Merck outside the submitted work. YH reports research grants from the UCB and Eli Lilly outside the submitted work. YH, NBN, and REC have a patent for a newer version of the tracer. REC is a consultant for Rodin Therapeutics and has received research funding from UCB. REC reports having received grants from AstraZeneca, Astellas, Eli Lilly, Pfizer, Taisho, and UCB, outside the submitted work. CHvD reports consulting fees from Kyowa Kirin, Roche, Merck, Eli Lilly, and Janssen and grants for clinical trials from Biogen, Novartis, Eli Lilly, Merck, Eisai, Janssen, Roche, Genentech, Toyama, and Biohaven, outside the submitted work. No other disclosures are reported.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

