Assessing seropositivity for IgG antibodies against SARS-CoV-2 in Ahmedabad city of India: a cross-sectional study
- PMID: 33402413
- PMCID: PMC7786546
- DOI: 10.1136/bmjopen-2020-044101
Assessing seropositivity for IgG antibodies against SARS-CoV-2 in Ahmedabad city of India: a cross-sectional study
Abstract
Objectives: To study the percentage seropositivity for SARS-CoV-2 to understand the pandemic status and predict the future situations in Ahmedabad.
Study design: Cross-sectional study.
Settings: Field area of Ahmedabad Municipal Corporation.
Participants: More than 30 000 individuals irrespective of their age, sex, acute/past COVID-19 infection participated in the serosurvey which covered all the 75 Urban Primary Health Centres (UPHCs) across 48 wards and 7 zones of the city. Study also involved healthcare workers (HCWs) from COVID-19/non-COVID-19 hospitals.
Interventions: Seropositivity of IgG antibodies against SARS-CoV-2 was measured as a mark of COVID-19 infection.
Primary and secondary outcomes: Seropositivity was used to calculate cumulative incidence. Correlation of seropositivity with available demographic detail was used for valid and precise assessment of the pandemic situation.
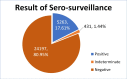
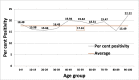
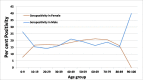
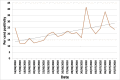
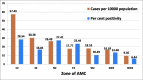
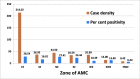
Results: From 30 054 samples, the results were available for 29 891 samples and the crude seropositivity is 17.61%. For all the various age groups, the seropositivity calculated between 15% and 20%. The difference in seropositivity for both the sex group is statistically not significant. The seropositivity is significantly lower (13.64%) for HCWs as compared with non-HCWs (18.71%). Seropositivity shows increasing trend with time. Zone with maximum initial cases has high positivity as compared with other zones. UPHCs with recent rise in cases are leading in seropositivity as compared with earlier and widely affected UPHCs.
Conclusions: The results of serosurveillance suggest that the population of Ahmedabad is still largely susceptible. People still need to follow preventive measures to protect themselves till an effective vaccine is available to the people at large. The data indicate the possibility of vanishing immunity over time and need further research to cross verify with scientific evidences.
Keywords: COVID-19; immunology; virology.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Population-based seropositivity for IgG antibodies against SARS-CoV-2 in Ahmedabad city.J Family Med Prim Care. 2021 Jun;10(6):2363-2368. doi: 10.4103/jfmpc.jfmpc_2062_20. Epub 2021 Jul 2. J Family Med Prim Care. 2021. PMID: 34322439 Free PMC article.
-
Difference in SARS-CoV-2 Antibody Status Between Patients With Cancer and Health Care Workers During the COVID-19 Pandemic in Japan.JAMA Oncol. 2021 Aug 1;7(8):1141-1148. doi: 10.1001/jamaoncol.2021.2159. JAMA Oncol. 2021. PMID: 34047762 Free PMC article.
-
Prevalence of IgG antibodies against SARS-CoV-2 among healthcare workers in a tertiary pediatric hospital in Poland.PLoS One. 2021 Apr 1;16(4):e0249550. doi: 10.1371/journal.pone.0249550. eCollection 2021. PLoS One. 2021. PMID: 33793673 Free PMC article.
-
Severe acute respiratory syndrome coronavirus 2 immunoglobulin G antibody: Seroprevalence among contacts of COVID-19 cases.Indian J Public Health. 2021 Jan-Mar;65(1):5-10. doi: 10.4103/ijph.IJPH_1199_20. Indian J Public Health. 2021. PMID: 33753682
-
COVID-Kavach-Based Seropositivity in the General Population of Ahmedabad: Just Before the Start of the Vaccination for the Elderly in India.Cureus. 2022 Mar 1;14(3):e22759. doi: 10.7759/cureus.22759. eCollection 2022 Mar. Cureus. 2022. PMID: 35371875 Free PMC article.
Cited by
-
Measles seroprevalence in persons over one year of age in Chandigarh, India.Hum Vaccin Immunother. 2022 Nov 30;18(6):2136453. doi: 10.1080/21645515.2022.2136453. Epub 2022 Oct 24. Hum Vaccin Immunother. 2022. PMID: 36279515 Free PMC article.
-
Seroprevalence of IgG and IgM Antibodies against SARS-CoV-2 Infection in Inhabitants of Itanagar Capital Region, Arunachal Pradesh, India.Maedica (Bucur). 2023 Mar;18(1):88-95. doi: 10.26574/maedica.2023.18.1.88. Maedica (Bucur). 2023. PMID: 37266471 Free PMC article.
-
The value of using COVID-19 antibody tests as a potential approach to prioritize vaccination delivery.PLoS One. 2024 Oct 16;19(10):e0311881. doi: 10.1371/journal.pone.0311881. eCollection 2024. PLoS One. 2024. PMID: 39413075 Free PMC article.
-
Seroprevalence of IgG antibodies against SARS-CoV-2 in India, March 2020 to August 2021: a systematic review and meta-analysis.Int J Infect Dis. 2022 Mar;116:59-67. doi: 10.1016/j.ijid.2021.12.353. Epub 2021 Dec 28. Int J Infect Dis. 2022. PMID: 34968773 Free PMC article.
-
The Global COVID-19 Pandemic Experience: Innovation Through Environmental Assessment and Seropositivity Surveillance.Int J Environ Res Public Health. 2025 Jul 18;22(7):1145. doi: 10.3390/ijerph22071145. Int J Environ Res Public Health. 2025. PMID: 40724210 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
