Neuron-Specific FMRP Roles in Experience-Dependent Remodeling of Olfactory Brain Innervation during an Early-Life Critical Period
- PMID: 33402421
- PMCID: PMC7888229
- DOI: 10.1523/JNEUROSCI.2167-20.2020
Neuron-Specific FMRP Roles in Experience-Dependent Remodeling of Olfactory Brain Innervation during an Early-Life Critical Period
Abstract
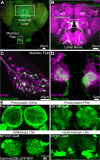
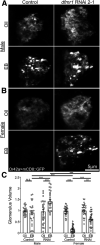
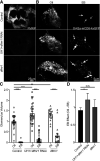
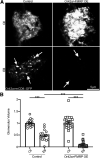
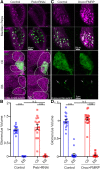
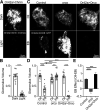
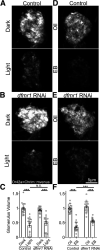
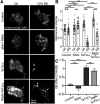
Critical periods are developmental windows during which neural circuits effectively adapt to the new sensory environment. Animal models of fragile X syndrome (FXS), a common monogenic autism spectrum disorder (ASD), exhibit profound impairments of sensory experience-driven critical periods. However, it is not known whether the causative fragile X mental retardation protein (FMRP) acts uniformly across neurons, or instead manifests neuron-specific functions. Here, we use the genetically-tractable Drosophila brain antennal lobe (AL) olfactory circuit of both sexes to investigate neuron-specific FMRP roles in the odorant experience-dependent remodeling of the olfactory sensory neuron (OSN) innervation during an early-life critical period. We find targeted OSN class-specific FMRP RNAi impairs innervation remodeling within AL synaptic glomeruli, whereas global dfmr1 null mutants display relatively normal odorant-driven refinement. We find both OSN cell autonomous and cell non-autonomous FMRP functions mediate odorant experience-dependent remodeling, with AL circuit FMRP imbalance causing defects in overall glomerulus innervation refinement. We find OSN class-specific FMRP levels bidirectionally regulate critical period remodeling, with odorant experience selectively controlling OSN synaptic terminals in AL glomeruli. We find OSN class-specific FMRP loss impairs critical period remodeling by disrupting responses to lateral modulation from other odorant-responsive OSNs mediating overall AL gain control. We find that silencing glutamatergic AL interneurons reduces OSN remodeling, while conversely, interfering with the OSN class-specific GABAA signaling enhances remodeling. These findings reveal control of OSN synaptic remodeling by FMRP with neuron-specific circuit functions, and indicate how neural circuitry can compensate for global FMRP loss to reinstate normal critical period brain circuit remodeling.SIGNIFICANCE STATEMENT Fragile X syndrome (FXS), the leading monogenic cause of intellectual disability and autism spectrum disorder (ASD), manifests severe neurodevelopmental delays. Likewise, FXS disease models display disrupted neurodevelopmental critical periods. In the well-mapped Drosophila olfactory circuit model, perturbing the causative fragile X mental retardation protein (FMRP) within a single olfactory sensory neuron (OSN) class impairs odorant-dependent remodeling during an early-life critical period. Importantly, this impairment requires activation of other OSNs, and the olfactory circuit can compensate when FMRP is removed from all OSNs. Understanding the neuron-specific FMRP requirements within a developing neural circuit, as well as the FMRP loss compensation mechanisms, should help us engineer FXS treatments. This work suggests FXS treatments could use homeostatic mechanisms to alleviate circuit-level deficits.
Keywords: Drosophila; critical period; fragile X mental retardation protein; fragile X syndrome; sensory experience; synapse elimination.
Copyright © 2021 the authors.
Figures

Similar articles
-
Activity-Dependent Remodeling of Drosophila Olfactory Sensory Neuron Brain Innervation during an Early-Life Critical Period.J Neurosci. 2019 Apr 17;39(16):2995-3012. doi: 10.1523/JNEUROSCI.2223-18.2019. Epub 2019 Feb 12. J Neurosci. 2019. PMID: 30755492 Free PMC article.
-
Neuron class-specific requirements for Fragile X Mental Retardation Protein in critical period development of calcium signaling in learning and memory circuitry.Neurobiol Dis. 2016 May;89:76-87. doi: 10.1016/j.nbd.2016.02.006. Epub 2016 Feb 3. Neurobiol Dis. 2016. PMID: 26851502 Free PMC article.
-
Fragile X Mental Retardation Protein Requirements in Activity-Dependent Critical Period Neural Circuit Refinement.Curr Biol. 2017 Aug 7;27(15):2318-2330.e3. doi: 10.1016/j.cub.2017.06.046. Epub 2017 Jul 27. Curr Biol. 2017. PMID: 28756946 Free PMC article.
-
Fragile X Mental Retardation Protein Regulates Activity-Dependent Membrane Trafficking and Trans-Synaptic Signaling Mediating Synaptic Remodeling.Front Mol Neurosci. 2018 Jan 12;10:440. doi: 10.3389/fnmol.2017.00440. eCollection 2017. Front Mol Neurosci. 2018. PMID: 29375303 Free PMC article. Review.
-
Molecular and cellular aspects of mental retardation in the Fragile X syndrome: from gene mutation/s to spine dysmorphogenesis.Adv Exp Med Biol. 2012;970:517-51. doi: 10.1007/978-3-7091-0932-8_23. Adv Exp Med Biol. 2012. PMID: 22351071 Review.
Cited by
-
Peripheral Fragile X messenger ribonucleoprotein is required for the timely closure of a critical period for neuronal susceptibility in the ventral cochlear nucleus.Front Cell Neurosci. 2023 May 25;17:1186630. doi: 10.3389/fncel.2023.1186630. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37305436 Free PMC article.
-
Neuron-to-glia and glia-to-glia signaling directs critical period experience-dependent synapse pruning.Front Cell Dev Biol. 2025 Feb 18;13:1540052. doi: 10.3389/fcell.2025.1540052. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40040788 Free PMC article. Review.
-
Deficits in olfactory system neurogenesis in neurodevelopmental disorders.Genesis. 2024 Apr;62(2):e23590. doi: 10.1002/dvg.23590. Genesis. 2024. PMID: 38490949 Free PMC article. Review.
-
Glia control experience-dependent plasticity in an olfactory critical period.bioRxiv [Preprint]. 2024 Oct 24:2024.07.05.602232. doi: 10.1101/2024.07.05.602232. bioRxiv. 2024. Update in: Elife. 2025 Jan 30;13:RP100989. doi: 10.7554/eLife.100989. PMID: 39005309 Free PMC article. Updated. Preprint.
-
Neuron-to-glia signaling drives critical period experience-dependent synapse pruning.Sci Rep. 2025 Jul 16;15(1):25744. doi: 10.1038/s41598-025-11528-3. Sci Rep. 2025. PMID: 40670566 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
