Sarcopenia may Influence the Prognosis in Advanced Thyroid Cancer Patients Treated With Molecular Targeted Therapy
- PMID: 33402489
- PMCID: PMC7880747
- DOI: 10.21873/invivo.12271
Sarcopenia may Influence the Prognosis in Advanced Thyroid Cancer Patients Treated With Molecular Targeted Therapy
Abstract
Background/aim: Reportedly, sarcopenia and nutritional status are associated with prognosis in cancer patients. However, data regarding the relationship of these factors with advanced thyroid cancer patients receiving molecular targeted therapy remains scarce. Therefore, we investigated the relationship between nutritional assessment, as well as sarcopenia, and prognosis in patients with advanced thyroid cancer undergoing molecular targeted therapy.
Patients and methods: In this retrospective study, sarcopenia and several markers of nutritional status were assessed in advanced thyroid cancer patients at the Kanazawa University Hospital, before the introduction of molecular targeted therapy.
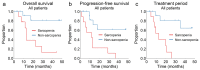
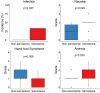
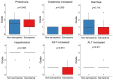
Results: Advanced thyroid cancer patients with sarcopenia presented a worse prognosis than those without sarcopenia. Additionally, sarcopenia strongly correlated with several markers of nutritional status, such as albumin, prognostic nutrition index, and Glasgow prognostic score.
Conclusion: Sarcopenia could be a prognostic factor in patients with advanced thyroid cancer receiving molecular targeted therapy.
Keywords: Sarcopenia; advanced thyroid cancer patient; molecular targeted therapy; nutritional assessment.
Copyright© 2021, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
SY obtained a research grant from Eisai Inc. Other Authors have no potential conflicts of interest.
Figures




References
-
- Incidence rate of thyroid cancer in Japan. 2017. Available at: https://www.env.go.jp/chemi/rhm/h29kisoshiryo/h29kiso-03-07-19.html [Last accessed 31th March 2017]
-
- Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smith JWA, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ, DECISION Investigators Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–328. doi: 10.1016/S0140-6736(14)60421-9. - DOI - PMC - PubMed
-
- Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–630. doi: 10.1056/NEJMoa1406470. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
