A review of the safety of vaccines used in routine immunization in Africa
- PMID: 33402911
- PMCID: PMC7750064
- DOI: 10.4314/ahs.v20i1.28
A review of the safety of vaccines used in routine immunization in Africa
Abstract
Background: Despite the significant role played by vaccines in global health, concerns over vaccine safety have increased tremendously over the years. There have been occasions where vaccines have caused rare, adverse reactions some of which have led to hospitalizations and even death. It is therefore important to establish the safety profile of routinely used vaccines in order to allay fears pertaining to their use.
Objectives: This review was aimed at pooling together the safety data of selected vaccines used for routine immunization in Africa, a region of the world with paucity of vaccine safety data.
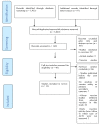
Methods: Adverse Events Following Immunization safety data was searched for rotavirus, yellow fever, measles, rubella, tuberculosis (Bacillus Calmette Guerin-BCG), pneumococcal, Haemophilus Influenza type b, polio, meningococcal and the influenza A (H1N1) vaccines in PUBMED, Google Scholar, Clinical trials.gov and Cochrane controlled register of trials databases.
Results: A total of twenty-four serious AEFIs and twenty-three minor AEFIs were identified from the review. The strength of association between AEFIs and vaccine was high for tuberculosis vaccine and moderate for all other vaccines.
Conclusion: Even though AEFIs (including mild and severe) were identified in the review, all the vaccines studied were generally well tolerated.
Keywords: Africa; Safety of vaccines; immunization.
© 2020 Yamoah P et al.
Similar articles
-
A phase III, open-label, randomised multicentre study to evaluate the immunogenicity and safety of a booster dose of two different reduced antigen diphtheria-tetanus-acellular pertussis-polio vaccines, when co-administered with measles-mumps-rubella vaccine in 3 and 4-year-old healthy children in the UK.Vaccine. 2018 Apr 19;36(17):2300-2306. doi: 10.1016/j.vaccine.2018.03.021. Epub 2018 Mar 22. Vaccine. 2018. PMID: 29576304 Clinical Trial.
-
Adverse Events Following Immunization in Brazil: Age of Child and Vaccine-Associated Risk Analysis Using Logistic Regression.Int J Environ Res Public Health. 2018 Jun 1;15(6):1149. doi: 10.3390/ijerph15061149. Int J Environ Res Public Health. 2018. PMID: 29865181 Free PMC article.
-
Vaccines for measles, mumps, rubella, and varicella in children.Cochrane Database Syst Rev. 2020 Apr 20;4(4):CD004407. doi: 10.1002/14651858.CD004407.pub4. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Nov 22;11:CD004407. doi: 10.1002/14651858.CD004407.pub5. PMID: 32309885 Free PMC article. Updated.
-
Immunogenicity and safety of a combined measles, mumps, rubella and varicella live vaccine (ProQuad ®) administered concomitantly with a booster dose of a hexavalent vaccine in 12-23-month-old infants.Vaccine. 2015 May 11;33(20):2379-86. doi: 10.1016/j.vaccine.2015.02.070. Epub 2015 Mar 9. Vaccine. 2015. PMID: 25765966 Clinical Trial.
-
Completeness and timeliness of diphtheria-tetanus-pertussis, measles-mumps-rubella, and polio vaccines in young children with chronic health conditions: A systematic review.Vaccine. 2019 Mar 22;37(13):1725-1735. doi: 10.1016/j.vaccine.2019.02.031. Epub 2019 Feb 25. Vaccine. 2019. PMID: 30814030
Cited by
-
Reproductive health issues, infections and non-communicable diseases.Afr Health Sci. 2020 Mar;20(1):I-IV. doi: 10.4314/ahs.v20i1.1. Afr Health Sci. 2020. PMID: 33402945 Free PMC article. No abstract available.
-
Safety of Vaccination within First Year of Life-The Experience of One General Medicine Center.Children (Basel). 2023 Jan 4;10(1):104. doi: 10.3390/children10010104. Children (Basel). 2023. PMID: 36670654 Free PMC article.
-
COVID-19 pandemic: SARS-CoV-2 specific vaccines and challenges, protection via BCG trained immunity, and clinical trials.Expert Rev Vaccines. 2021 Jul;20(7):857-880. doi: 10.1080/14760584.2021.1938550. Epub 2021 Jun 15. Expert Rev Vaccines. 2021. PMID: 34078215 Free PMC article. Review.
-
Pharmacovigilance for Vaccines Used in Pregnancy: A Gap Analysis From Uganda.Pediatr Infect Dis J. 2025 Feb 1;44(2S):S123-S129. doi: 10.1097/INF.0000000000004705. Epub 2025 Feb 14. Pediatr Infect Dis J. 2025. PMID: 39951089 Free PMC article.
-
Assessment of adherence to pre-vaccination precautions and AEFI reporting practices during BCG vaccination in 4 hospitals in Ghana.Hum Vaccin Immunother. 2023 Dec 31;19(1):2199654. doi: 10.1080/21645515.2023.2199654. Hum Vaccin Immunother. 2023. PMID: 37127290 Free PMC article.
References
-
- Larson HJ, Cooper LZ, Eskola J, Katz SL, Ratzan S. Addressing the vaccine confidence gap. The Lancet. 2011;378:526–535. PubMed. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical