Nivolumab treatment in advanced non-small cell lung cancer: real-world long-term outcomes within overall and special populations (the UNIVOC study)
- PMID: 33403011
- PMCID: PMC7745546
- DOI: 10.1177/1758835920967237
Nivolumab treatment in advanced non-small cell lung cancer: real-world long-term outcomes within overall and special populations (the UNIVOC study)
Abstract
Objective: To describe long-term outcomes of patients treated with nivolumab for advanced non-small cell lung cancer (aNSCLC) in everyday clinical practice in France, with a focus on patients aged ⩾80 years, patients with renal impairment and patients with brain metastases.
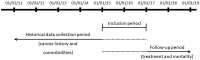
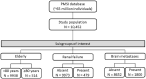
Methods: The study included all patients with aNSCLC recorded in the French national hospital database, starting nivolumab in 2015-2016 and followed until December 2018. Patients were stratified by age, the presence of renal impairment and brain metastasis, as documented in the hospital discharge summaries. Information was retrieved on demographics, comorbidities and treatment history at baseline. Time to discontinuation of nivolumab treatment and overall survival were estimated using Kaplan-Meier survival analysis.
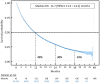
Results: Overall, 10,452 patients were included, of whom 514 were octogenarians, 479 had renal impairment and 1800 had brain metastases at baseline. Median duration of nivolumab treatment was 2.8 months in the overall population and in both the octogenarian and renally impaired subgroups, and 2.3 months in patients with brain metastases. Median overall survival in these patient groups was 11.7 months (95% confidence interval: 11.3-12.2), 11.7 months (11.3-12.1), 11.7 months (11.3-12.2) and 9.9 months (9.0-10.9) respectively. Three-year overall survival rates were 19.1% (18.1-20.2) in the overall population, 16.5% (11.6-23.4) in octogenarians, 15.9% (11.8-21.4) in patients with renal impairment and 21.7% (19.4-24.2) in those with brain metastases.
Conclusion: This large nationwide retrospective real-life cohort provided narrow estimates of long-term overall survival, which reached 19% at 3 years, consistent with data from phase III trials of nivolumab. Survival rates were comparable in the three special populations of interest and the overall population.
Keywords: SNDS; brain metastases; elderly; immunotherapy; nivolumab; non-small cell lung cancer; renal impairment.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: JBA was supported by grants from Fondation pour la Recherche Médicale (FRM). CC reports consultancy fees from Astra Zeneca, Boehringer Ingelheim, MSD, Pierre Fabre Oncology, Lilly, Roche, Bristol- Myers Squibb, Novartis, Lilly, Pierre Fabre Oncology and Boehringer Ingelheim. RC reports consultancy fees from Astra-Zeneca, Bristol- Myers Squibb, Roche and Takeda. MGL reports consultancy fees/research funding from Bristol-Myers Squibb, Astra Zeneca, MSD, Roche and Novartis. FEC, CYC, and AFG are employed by Bristol Myers Squibb. BJ and RJ are employees of HEVA.
Figures

References
-
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29: iv192–iv237. - PubMed
-
- Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 2018; 62: 29–39. - PubMed
LinkOut - more resources
Full Text Sources

