Efficacy and safety of apatinib as third- or further-line therapy for patients with advanced NSCLC: a retrospective study
- PMID: 33403012
- PMCID: PMC7745562
- DOI: 10.1177/1758835920968472
Efficacy and safety of apatinib as third- or further-line therapy for patients with advanced NSCLC: a retrospective study
Abstract
Background: Apatinib, an oral small-molecule angiogenesis inhibitor, selectively inhibits vascular endothelial growth factor receptor 2 (VEGFR-2), which inhibits vascular endothelial growth factor (VEGF) stimulated endothelial cell migration and proliferation and decreases tumour growth and metastasis. Recently, the efficacy of multi-target angiogenic drugs has been demonstrated for many cancers, including non-small-cell lung cancer (NSCLC). The aim of this retrospective study was to evaluate the clinical efficacy of apatinib in patients with advanced NSCLC.
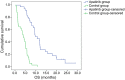
Patients and methods: We conducted a retrospective analysis of 70 patients with advanced NSCLC who received second-line and later treatment from November 2015 to July 2017 with poor results. Out of the 70 patients, 36 patients received apatinib treatment after second-line or later treatment, whereas 34 patients in the control group did not receive further treatment. The patients were treated with oral apatinib 500 mg once a day every day for 4 weeks per cycle. Treatment was continued in responding and stable patients until disease progression or intolerable toxicity. The objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and side effects of the drug were recorded and reviewed.
Results: ORR, DCR, PFS, and OS were evaluated in 36 patients receiving apatinib and 34 patients in the control group. The ORR and DCR in patients receiving apatinib therapy were 22.2% and 77.8%, respectively. The median PFS and OS in the treatment group were 5.6 and 9.6 months, respectively. The median OS in the apatinib group was significantly longer than that in the control group (9.6 versus 3.8 months; p < 0.0001). In contrast, there were no differences in adverse reactions between the patients in the treatment and control groups.
Conclusion: Apatinib showed favourable efficacy and safety and can thus be used as a treatment option for patients with advanced NSCLC.
Keywords: TKI; angiogenesis; anti-VEGF; apatinib; efficacy; non-small-cell lung cancer; safety.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures



References
-
- Shi Y, Sun Y, Yu J, et al. China experts consensus on the diagnosis and treatment of advanced stage primary lung cancer (2016 version). Asia Pac J Clin Oncol 2017; 13: 87–103. - PubMed
-
- Xue C, Hu Z, Jiang W, et al. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer 2012; 77: 371–375. - PubMed
LinkOut - more resources
Full Text Sources

