Drug Repurposing for Prevention and Treatment of COVID-19: A Clinical Landscape
- PMID: 33403227
- PMCID: PMC7758544
- DOI: 10.15190/d.2020.18
Drug Repurposing for Prevention and Treatment of COVID-19: A Clinical Landscape
Abstract
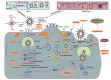
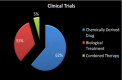
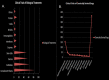
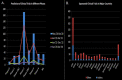
SARS-CoV-2, the novel coronavirus strain responsible for the current pandemic of COVID-19, has rendered the entire humanity suffering. Several months have passed since the pandemic has struck. However, the world is still looking for an effective treatment plan to battle the viral infection. The first vaccine just received emergency approval in December 2020 for use in USA and UK. These are excellent news, however, the worldwide distribution of such vaccine, the possibility of virus mutation and the lack of data regarding the long-term effects of such vaccines are a significant concern. In addition, although remdesivir was recently approved by the FDA to be used as a clinical drug against COVID-19, it hasn't stood out yet as a proven form of therapeutics. Such inability to produce a novel therapy has caused enough inconveniences for the affected people worldwide. Repurposing the already available drugs to fight against the virus seems to be a reasonable option amidst such uncertainty. Given the vast collection of potential treatment candidates to be explored against COVID-19, there is a decent chance that a success in this regard will serve the intermediary purpose of clinically treating the infection until a COVID-19 vaccine is widely distributed worldwide and will be able to treat COVID-19 patients that do not adequately respond to vaccines. Such treatments may prove very useful in future coronavirus outbreaks too. Proper research into these repurposing treatments may yield a certain insight into the field of novel treatment production as well. This review study accumulates a relevant set of information about drugs and vaccines against COVID-19, in terms of their repurposing properties and the specific phases of clinical trials they are undergoing across the world. A potential timeline is also suggested to estimate when an effective result can be expected from the ongoing clinical trials for a better anticipation of the drug landscape. This study will hopefully help accelerate investment of resources into development and discovery of drugs and vaccines against the infection.
Keywords: COVID-19; SARS-CoV-2; clinical trial; drug repositioning; phases; therapeutics; vaccine.
Copyright © 2020, Applied Systems.
Conflict of interest statement
Conflict of interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Author have no conflicts of interest to declare.
Figures
References
-
- A Novel Coronavirus from Patients with Pneumonia in China, 2019. Zhu Na, Zhang Dingyu, Wang Wenling, Li Xingwang, Yang Bo, Song Jingdong, Zhao Xiang, Huang Baoying, Shi Weifeng, Lu Roujian, Niu Peihua, Zhan Faxian, Ma Xuejun, Wang Dayan, Xu Wenbo, Wu Guizhen, Gao George F, Tan Wenjie. The New England journal of medicine. 2020;382(8):727–733. - PMC - PubMed
-
- Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Dyall Julie, Coleman Christopher M, Hart Brit J, Venkataraman Thiagarajan, Holbrook Michael R, Kindrachuk Jason, Johnson Reed F, Olinger Gene G, Jahrling Peter B, Laidlaw Monique, Johansen Lisa M, Lear-Rooney Calli M, Glass Pamela J, Hensley Lisa E, Frieman Matthew B. Antimicrobial agents and chemotherapy. 2014;58(8):4885–93. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
