Pharmacotherapeutics of SARS-CoV-2 Infections
- PMID: 33403500
- PMCID: PMC7785334
- DOI: 10.1007/s11481-020-09968-x
Pharmacotherapeutics of SARS-CoV-2 Infections
Abstract
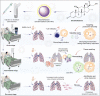
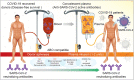
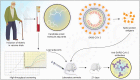
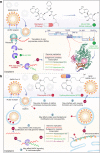
The COVID-19 pandemic has affected more than 38 million people world-wide by person to person transmission of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Therapeutic and preventative strategies for SARS-CoV-2 remains a significant challenge. Within the past several months, effective treatment options have emerged and now include repurposed antivirals, corticosteroids and virus-specific antibodies. The latter has included convalescence plasma and monoclonal antibodies. Complete viral eradication will be achieved through an effective, safe and preventative vaccine. To now provide a comprehensive summary for each of the pharmacotherapeutics and preventative strategies being offered or soon to be developed for SARS-CoV-2.
Keywords: Antibodies; Antivirals; COVID-19 therapeutics; SARS-CoV-2; Vaccine.
Figures


References
-
- Alpert A, Pickman Y, Leipold M, Rosenberg-Hasson Y, Ji X, Gaujoux R, Rabani H, Starosvetsky E, Kveler K, Schaffert S, Furman D, Caspi O, Rosenschein U, Khatri P, Dekker CL, Maecker HT, Davis MM, Shen-Orr SS. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med. 2019;25:487–495. doi: 10.1038/s41591-019-0381-y. - DOI - PMC - PubMed
-
- American Scientific (2020) Coronavirus Vaccines May Not Work for the Elderly—and This Lab Aims to Change That. . https://www.scientificamerican.com/article/coronavirus-vaccines-may-not-...
-
- Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, Taylor J, Spicer K, Bardossy AC, Oakley LP, Tanwar S, Dyal JW, Harney J, Chisty Z, Bell JM, Methner M, Paul P, Carlson CM, McLaughlin H, Thornburg N, Tong S, Tamin A, Tao Y, Uehara A, Harcourt J, Clark S, Brostrom-Smith C, Page LC, Kay M, Lewis J, Montgomery P, Stone ND, Clark TA, Honein MA, Duchin JS, Jernigan JA, Public Health–Seattle and King County and CDC COVID-19 Investigation Team Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

