Inhaled ciclesonide is efficacious and well tolerated in the treatment of severe equine asthma in a large prospective European clinical trial
- PMID: 33403727
- PMCID: PMC8518630
- DOI: 10.1111/evj.13419
Inhaled ciclesonide is efficacious and well tolerated in the treatment of severe equine asthma in a large prospective European clinical trial
Abstract
Background: Ciclesonide is a glucocorticoid prodrug, already registered for human use. Due to its mode of action and inhaled route of administration, it was considered an appropriate treatment option for horses with severe equine asthma. Although the efficacy of inhaled ciclesonide has been demonstrated in horses with asthma exacerbations under controlled mouldy hay challenge conditions, it has not yet been reported under field conditions.
Objectives: To assess the effectiveness and safety of inhaled ciclesonide for the treatment of severe equine asthma.
Study design: Prospective, multicentre, placebo-controlled, randomised, double-blinded study.
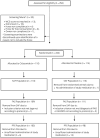
Methods: Two-hundred and twenty-four client-owned horses with severe equine asthma were randomised (1:1 ratio) to receive either ciclesonide inhalation (343 µg/actuation) solution or placebo (0 µg/actuation). Treatments (placebo or ciclesonide) were administered with a nonpressurised Soft Mist™ inhaler specifically developed for horses (Aservo® EquiHaler® ) at doses of 8 actuations twice daily for the first 5 days and 12 actuations once daily for the following 5 days. Primary outcome was a success/failure analysis with the a priori definition of treatment success as a 30% or greater reduction in weighted clinical score (WCS) between Day 0 and Day 10 (±1).
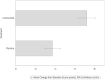
Results: The treatment success rate (as defined above) in ciclesonide-treated horses was 73.4% (80/109) after 10 (±1) days of treatment, being significantly higher than in the placebo group with 43.2% (48/111; P < 0.0001). Few systemic and local adverse events of ciclesonide were observed.
Main limitations: The severity of clinical signs of severe equine asthma varies over time; despite the prohibition of environmental management changes during the study, a placebo effect was also identified. This potentially contributed, in part, to the clinical improvement observed in the ciclesonide-treated group.
Conclusions: Ciclesonide inhalation solution administered by the Aservo® EquiHaler® effectively reduced severity of clinical signs in a majority of horses with severe equine asthma and was well tolerated.
Keywords: ciclesonide; clinical trial; cough; equine asthma; heaves; horse; inhaled corticosteroids.
© 2021 Boehringer Ingelheim Vetmedica GmbH. Equine Veterinary Journal published by John Wiley & Sons Ltd on behalf of EVJ Ltd.
Conflict of interest statement
B. Albrecht, O. Engel and M. von Salis‐Soglio are employees of Boehringer Ingelheim Vetmedica GmbH, the marketing authorisation holder of Aservo® Equihaler® with ciclesonide as active ingredient. H.‐W. Mueller is an employee of Boehringer Ingelheim Pharma GmbH & Co KG. R.S. Pirie has acted as consultant to Boehringer Ingelheim Vetmedica GmbH. B. Albrecht is co‐inventor on a patent regarding the use of ciclesonide in horses.
Figures


Similar articles
-
Effect of different doses of inhaled ciclesonide on lung function, clinical signs related to airflow limitation and serum cortisol levels in horses with experimentally induced mild to severe airway obstruction.Equine Vet J. 2019 Nov;51(6):779-786. doi: 10.1111/evj.13093. Epub 2019 Apr 5. Equine Vet J. 2019. PMID: 30854685 Free PMC article.
-
Use of inhaled ciclesonide for treatment of moderate asthma in Thoroughbred racehorses.J Vet Intern Med. 2025 Mar-Apr;39(2):e17267. doi: 10.1111/jvim.17267. J Vet Intern Med. 2025. PMID: 39945569 Free PMC article.
-
LC-HRMS/MS study of the prodrug ciclesonide and its active metabolite desisobutyryl-ciclesonide in plasma after an inhalative administration to horses for doping control purposes.Drug Test Anal. 2022 Feb;14(2):252-261. doi: 10.1002/dta.3174. Epub 2021 Oct 30. Drug Test Anal. 2022. PMID: 34634175
-
Ciclesonide: a novel inhaled corticosteroid for asthma.Drugs Today (Barc). 2004 Jul;40(7):569-76. doi: 10.1358/dot.2004.40.7.850475. Drugs Today (Barc). 2004. PMID: 15510231 Review.
-
[Ciclesonide -- a new inhaled corticosteroid].Pneumologie. 2005 Oct;59(10):689-95. doi: 10.1055/s-2005-915570. Pneumologie. 2005. PMID: 16222581 Review. German.
Cited by
-
Decision Making in Severe Equine Asthma-Diagnosis and Monitoring.Animals (Basel). 2023 Dec 16;13(24):3872. doi: 10.3390/ani13243872. Animals (Basel). 2023. PMID: 38136909 Free PMC article. Review.
-
Protein microarray allergen profiling in bronchoalveolar lavage fluid and serum of horses with asthma.J Vet Intern Med. 2023 Jan;37(1):328-337. doi: 10.1111/jvim.16600. Epub 2022 Dec 8. J Vet Intern Med. 2023. PMID: 36479920 Free PMC article.
-
Inhalative Nanoparticulate CpG Immunotherapy in Severe Equine Asthma: An Innovative Therapeutic Concept and Potential Animal Model for Human Asthma Treatment.Animals (Basel). 2022 Aug 16;12(16):2087. doi: 10.3390/ani12162087. Animals (Basel). 2022. PMID: 36009677 Free PMC article. Review.
-
Respiratory Rate Recovery After Submaximal Lunging Exercise Is Delayed in Asthmatic Horses with Neutrophilic Airway Inflammation.Animals (Basel). 2025 Mar 2;15(5):713. doi: 10.3390/ani15050713. Animals (Basel). 2025. PMID: 40075996 Free PMC article.
-
Advanced Strategies of Drug Delivery via Oral, Topical, and Parenteral Administration Routes: Where Do Equine Medications Stand?Pharmaceutics. 2023 Jan 4;15(1):186. doi: 10.3390/pharmaceutics15010186. Pharmaceutics. 2023. PMID: 36678815 Free PMC article. Review.
References
-
- Lavoie JP. Is the time primed for equine asthma? Equine Vet Educ. 2015;27:225–6.
-
- Pirie RS, Couetil LL, Robinson NE, Lavoie JP. Equine asthma: an appropriate, translational and comprehendible terminology? Equine Vet J. 2016;48:403–5. - PubMed
-
- Bullone M, Lavoie JP. Asthma "of horses and men"‐How can equine heaves help us better understand human asthma immunopathology and its functional consequences? Mol Immunol. 2015;66:97–105. - PubMed
-
- Couetil LL, Chilcoat CD, DeNicola DB, Clark SP, Glickman NW, Glickman LT. Randomized, controlled study of inhaled fluticasone propionate, oral administration of prednisone, and environmental management of horses with recurrent airway obstruction. Am J Vet Res. 2005;66:1665–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

