Ruthenium-Catalyzed Dehydrogenation Through an Intermolecular Hydrogen Atom Transfer Mechanism
- PMID: 33403774
- PMCID: PMC8048662
- DOI: 10.1002/anie.202015837
Ruthenium-Catalyzed Dehydrogenation Through an Intermolecular Hydrogen Atom Transfer Mechanism
Abstract
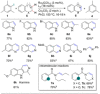
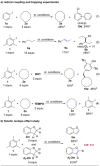
The direct dehydrogenation of alkanes is among the most efficient ways to access valuable alkene products. Although several catalysts have been designed to promote this transformation, they have unfortunately found limited applications in fine chemical synthesis. Here, we report a conceptually novel strategy for the catalytic, intermolecular dehydrogenation of alkanes using a ruthenium catalyst. The combination of a redox-active ligand and a sterically hindered aryl radical intermediate has unleashed this novel strategy. Importantly, mechanistic investigations have been performed to provide a conceptual framework for the further development of this new catalytic dehydrogenation system.
Keywords: alkenes; aryl radicals; dehydrogenation; hydrogenation atom transfer; redox-active ligands.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
A New Paradigm in Pincer Iridium Chemistry: PCN Complexes for (De)Hydrogenation Catalysis and Beyond.Acc Chem Res. 2022 Aug 2;55(15):2148-2161. doi: 10.1021/acs.accounts.2c00311. Epub 2022 Jul 19. Acc Chem Res. 2022. PMID: 35852837
-
Ruthenium-Catalyzed Ammonia Borane Dehydrogenation: Mechanism and Utility.Acc Chem Res. 2017 Jan 17;50(1):86-95. doi: 10.1021/acs.accounts.6b00482. Epub 2016 Dec 29. Acc Chem Res. 2017. PMID: 28032510
-
Hydrogenation of Alkenes via Cooperative Hydrogen Atom Transfer.J Am Chem Soc. 2020 Nov 11;142(45):19316-19326. doi: 10.1021/jacs.0c09544. Epub 2020 Oct 29. J Am Chem Soc. 2020. PMID: 33119986
-
Recent advances in osmium-catalyzed hydrogenation and dehydrogenation reactions.Acc Chem Res. 2015 Feb 17;48(2):363-79. doi: 10.1021/ar5003818. Epub 2015 Feb 4. Acc Chem Res. 2015. PMID: 25650714 Review.
-
Dehydrogenative Pd and Ni Catalysis for Total Synthesis.Acc Chem Res. 2021 Mar 2;54(5):1118-1130. doi: 10.1021/acs.accounts.0c00787. Epub 2021 Feb 16. Acc Chem Res. 2021. PMID: 33592147 Free PMC article. Review.
Cited by
-
Tandem dehydrogenation-olefination-decarboxylation of cycloalkyl carboxylic acids via multifold C-H activation.Nat Commun. 2024 Jun 25;15(1):5370. doi: 10.1038/s41467-024-49359-x. Nat Commun. 2024. PMID: 38918374 Free PMC article.
-
Cobalt-catalyzed chemoselective dehydrogenation through radical translocation under visible light.Chem Sci. 2022 Jun 15;13(26):7947-7954. doi: 10.1039/d2sc02291e. eCollection 2022 Jul 6. Chem Sci. 2022. PMID: 35865906 Free PMC article.
-
Multiple remote C(sp3)-H functionalizations of aliphatic ketones via bimetallic Cu-Pd catalyzed successive dehydrogenation.Chem Sci. 2022 Nov 14;13(46):13843-13850. doi: 10.1039/d2sc05370e. eCollection 2022 Nov 30. Chem Sci. 2022. PMID: 36544736 Free PMC article.
-
Selective desaturation of amides: a direct approach to enamides.Chem Sci. 2022 Jul 6;13(31):9056-9061. doi: 10.1039/d2sc02210a. eCollection 2022 Aug 10. Chem Sci. 2022. PMID: 36091215 Free PMC article.
-
Homogenous Cr and C Doped 3D Self-Supporting NiO Cellular Nanospheres for Hydrogen Evolution Reaction.Materials (Basel). 2022 Oct 13;15(20):7120. doi: 10.3390/ma15207120. Materials (Basel). 2022. PMID: 36295190 Free PMC article.
References
-
- None
-
- “The Alkenes: Volumes 1 and 2”: PATAI's Chemistry of Functional Groups (Eds.: Patai S., Zabicky J.), Wiley, New York, 1964/1970;
-
- Weissermel K., Arpe H.-J., Olefins, Industrial Organic Chemistry, Wiley-VCH, Weinheim, 2008, p. 59.
-
- None
-
- Labinger J. A., Bercaw J. E., Nature 2002, 417, 507; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

