Identification of Conformational B-cell Epitopes in Diphtheria Toxin at Varying Temperatures Using Molecular Dynamics Simulations
- PMID: 33403838
- PMCID: PMC8410147
- DOI: 10.22092/ari.2019.127251.1377
Identification of Conformational B-cell Epitopes in Diphtheria Toxin at Varying Temperatures Using Molecular Dynamics Simulations
Abstract
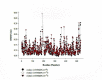
The changes in temperature levels can potentially affect the toxins in terms of stability and immunological properties via alteration of their structures. Diphtheria Toxin (DT) is highly considered by scientists since its mechanism of action is similar to those of most bacterial toxins, such as botulinum, tetanus, and anthrax. The protection of conformational B-cell epitopes is critically important in the process of diphtheria vaccine production. This study aimed to evaluate the conformational changes of the DT structure at three different temperature levels (27˚C, 37˚C, and 47˚C) using molecular dynamic simulations. Secondary structures were analyzed in YASARA software. According to the results, significant decreases were observed in percentages of the β-sheets, turns, and the helices of the DT structure at 47˚C in comparison with those at 27˚C and 37˚C. Furthermore, the tertiary structure of the DT was compared at different temperatures using the contact map. Accordingly, the results showed that the root-mean-square deviation of the DT structure increased upon temperature rising. In addition, amino acids D68, G128, G171, C186, and K534-S535 at 27˚C and 37˚C, as well as amino acids G26, P38, S291, T267, H384, A356, and V518 at 47˚C showed higher root mean square fluctuation values. The finding demonstrated that the stability of the DT structure decreased at high temperature (47˚C). The solvent-accessible surface area diagram showed that the hydrophobicity of the DT structure increased via temperature rising, and the amino acid residues belonging to B-cell epitopes extended through increasing temperature. However, B-cell epitopes belonging to the junction region of chains A and B were only present at 37˚C. The results of this study are expected to be applicable for determining a suitable temperature level for the production process of the diphtheria vaccine.
Keywords: B-cell epitope; Diphtheria toxin; Molecular dynamics simulation; Stability; Temperature.
Copyright © 2021, Author(s). Published by Kowsar.
Conflict of interest statement
We declare no conflict of interest.
Figures

References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015; 1:19–25.
-
- Arfken G. The method of steepest descents. Math Methods Phys. 1985; 3: 428–436.
-
- Audibert F, Jolivet M, Chedid L, Alouf JE, Boquet P, Rivaille P, Siffert O. Active antitoxic immunization by a diphtheria toxin synthetic oligopeptide. Nature. 1981;289(5798):593–4. - PubMed
-
- Boquet P, Alouf JE, Duflot E, Siffert O, Rivaille P. Characteristics of guinea-pig immune sera elicited by a synthetic diphtheria toxin oligopeptide. Mol Immunol. 1982;19(12):1541–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
