Current Models for Development of Disease-Modifying Osteoarthritis Drugs
- PMID: 33403944
- PMCID: PMC8098772
- DOI: 10.1089/ten.TEC.2020.0309
Current Models for Development of Disease-Modifying Osteoarthritis Drugs
Abstract
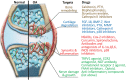
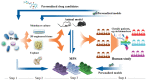
Osteoarthritis (OA) is a painful and disabling disease that affects millions of people worldwide. Symptom-alleviating treatments exist, although none with long-term efficacy. Furthermore, there are currently no disease-modifying OA drugs (DMOADs) with demonstrated efficacy in OA patients, which is, in part, attributed to a lack of full understanding of the pathogenesis of OA. The inability to translate findings from basic research to clinical applications also highlights the deficiencies in the available OA models at simulating the clinically relevant pathologies and responses to treatments in humans. In this review, the current status in the development of DMOADs will be first presented, with special attention to those in Phase II-IV clinical trials. Next, current in vitro, ex vivo, and in vivo OA models are summarized and the respective advantages and disadvantages of each are highlighted. Of note, the development and application of microphysiological or tissue-on-a-chip systems for modeling OA in humans are presented and the issues that need to be addressed in the future are discussed. Microphysiological systems should be given serious consideration for their inclusion in the DMOAD development pipeline, both for their ability to predict drug safety and efficacy in human clinical trials at present, as well as for their potential to serve as a test platform for personalized medicine. Impact statement At present, no disease-modifying osteoarthritis (OA) drugs (DMOADs) have been approved for widespread clinical use by regulatory bodies. The failure of developing effective DMOADs is likely owing to multiple factors, not the least of which are the intrinsic differences between the intact human knee joint and the preclinical models. This work summarizes the current OA models for the development of DMOADs, discusses the advantages/disadvantages of each, and then proposes future model development to aid in the discovery of effective and personalized DMOADs. The review also highlights the microphysiological systems, which are emerging as a new platform for drug development.
Keywords: disease-modifying osteoarthritis drugs; microphysiological system; models; osteoarthritis; personalized medicine; tissue-on-a-chip.
Conflict of interest statement
No competing financial interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

