Nanoscale Hydration in Layered Manganese Oxides
- PMID: 33404244
- PMCID: PMC7880569
- DOI: 10.1021/acs.langmuir.0c02592
Nanoscale Hydration in Layered Manganese Oxides
Abstract
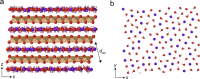
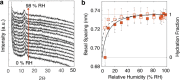
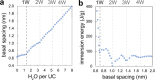
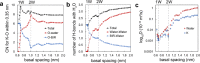
Birnessite is a layered MnO2 mineral capable of intercalating nanometric water films in its bulk. With its variable distributions of Mn oxidation states (MnIV, MnIII, and MnII), cationic vacancies, and interlayer cationic populations, birnessite plays key roles in catalysis, energy storage solutions, and environmental (geo)chemistry. We here report the molecular controls driving the nanoscale intercalation of water in potassium-exchanged birnessite nanoparticles. From microgravimetry, vibrational spectroscopy, and X-ray diffraction, we find that birnessite intercalates no more than one monolayer of water per interlayer when exposed to water vapor at 25 °C, even near the dew point. Molecular dynamics showed that a single monolayer is an energetically favorable hydration state that consists of 1.33 water molecules per unit cell. This monolayer is stabilized by concerted potassium-water and direct water-birnessite interactions, and involves negligible water-water interactions. Using our composite adsorption-condensation-intercalation model, we predicted humidity-dependent water loadings in terms of water intercalated in the internal and adsorbed at external basal faces, the proportions of which vary with particle size. The model also accounts for additional populations condensed on and between particles. By describing the nanoscale hydration of birnessite, our work secures a path for understanding the water-driven catalytic chemistry that this important layered manganese oxide mineral can host in natural and technological settings.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Chukhrov F. V.; Gorshkov A. I.; Rudnitskaya E. S.; Beresovskaya V. V.; Sivtsov A. V. Manganese Minerals in Clays: A Review. Clays Clay Miner. 1980, 28, 346–354. 10.1346/ccmn.1980.0280504. - DOI
-
- McKenzie R.Manganese Oxides and Hydroxides. In Minerals in Soil Environments, 2 ed.; Dixon J. B., Weed S. B., Eds.; Soil Science Society of America: Madison, Wisconsin, USA, 1989; Vol. 1, pp 439–465.
-
- Johnson E. A.; Post J. E. Water in the interlayer region of birnessite: Importance in cation exchange and structural stability. Am. Miner. 2006, 91, 609–618. 10.2138/am.2006.2090. - DOI
-
- Villalobos M.; Carrillo-Cárdenas M.; Gibson R.; López-Santiago N. R.; Morales J. A. The influence of particle size and structure on the sorption and oxidation behaviour of birnessite: II. Adsorption and oxidation of four polycyclic aromatic hydrocarbons. Environ. Chem. 2014, 11, 279–288. 10.1071/en13161. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

