Autophagy inhibition rescues structural and functional defects caused by the loss of mitochondrial chaperone Hsc70-5 in Drosophila
- PMID: 33404278
- PMCID: PMC8526020
- DOI: 10.1080/15548627.2020.1871211
Autophagy inhibition rescues structural and functional defects caused by the loss of mitochondrial chaperone Hsc70-5 in Drosophila
Abstract
We investigated in larval and adult Drosophila models whether loss of the mitochondrial chaperone Hsc70-5 is sufficient to cause pathological alterations commonly observed in Parkinson disease. At affected larval neuromuscular junctions, no effects on terminal size, bouton size or number, synapse size, or number were observed, suggesting that we studied an early stage of pathogenesis. At this stage, we noted a loss of synaptic vesicle proteins and active zone components, delayed synapse maturation, reduced evoked and spontaneous excitatory junctional potentials, increased synaptic fatigue, and cytoskeleton rearrangements. The adult model displayed ATP depletion, altered body posture, and susceptibility to heat-induced paralysis. Adult phenotypes could be suppressed by knockdown of dj-1β, Lrrk, DCTN2-p50, DCTN1-p150, Atg1, Atg101, Atg5, Atg7, and Atg12. The knockdown of components of the macroautophagy/autophagy machinery or overexpression of human HSPA9 broadly rescued larval and adult phenotypes, while disease-associated HSPA9 variants did not. Overexpression of Pink1 or promotion of autophagy exacerbated defects.Abbreviations: AEL: after egg laying; AZ: active zone; brp: bruchpilot; Csp: cysteine string protein; dlg: discs large; eEJPs: evoked excitatory junctional potentials; GluR: glutamate receptor; H2O2: hydrogen peroxide; mEJP: miniature excitatory junctional potentials; MT: microtubule; NMJ: neuromuscular junction; PD: Parkinson disease; Pink1: PTEN-induced putative kinase 1; PSD: postsynaptic density; SSR: subsynaptic reticulum; SV: synaptic vesicle; VGlut: vesicular glutamate transporter.
Keywords: Atg1; Hsc70-5; microtubule; mitochondria; mitophagy; rapamycin; synapse.
Conflict of interest statement
All authors declare no conflict of interest.
Figures


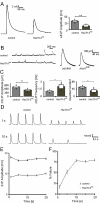
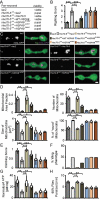
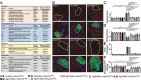
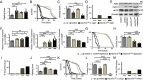


References
-
- Dauer W, Przedborski S.. Parkinson’s disease: mechanisms and models. Neuron. 2003. Sep 11;39(6):889–909. - PubMed
-
- Picconi B, Piccoli G, Calabresi P. Synaptic dysfunction in Parkinson’s disease. Adv Exp Med Biol. 2012;970:553–572. - PubMed
-
- Bras J, Guerreiro R, SnapShot: HJ. Genetics of Parkinson’s disease. Cell. 2015. Jan 29;160(3):570–570 e1. - PubMed
-
- Miura E, Hasegawa T, Konno M, et al. VPS35 dysfunction impairs lysosomal degradation of alpha-synuclein and exacerbates neurotoxicity in a Drosophila model of Parkinson’s disease. Neurobiol Dis. 2014. Nov;71:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
