Bacterial symbiont subpopulations have different roles in a deep-sea symbiosis
- PMID: 33404502
- PMCID: PMC7787665
- DOI: 10.7554/eLife.58371
Bacterial symbiont subpopulations have different roles in a deep-sea symbiosis
Abstract
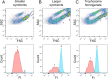
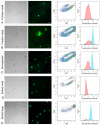
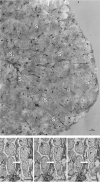
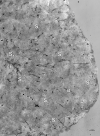
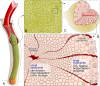
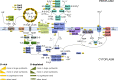
The hydrothermal vent tubeworm Riftia pachyptila hosts a single 16S rRNA phylotype of intracellular sulfur-oxidizing symbionts, which vary considerably in cell morphology and exhibit a remarkable degree of physiological diversity and redundancy, even in the same host. To elucidate whether multiple metabolic routes are employed in the same cells or rather in distinct symbiont subpopulations, we enriched symbionts according to cell size by density gradient centrifugation. Metaproteomic analysis, microscopy, and flow cytometry strongly suggest that Riftia symbiont cells of different sizes represent metabolically dissimilar stages of a physiological differentiation process: While small symbionts actively divide and may establish cellular symbiont-host interaction, large symbionts apparently do not divide, but still replicate DNA, leading to DNA endoreduplication. Moreover, in large symbionts, carbon fixation and biomass production seem to be metabolic priorities. We propose that this division of labor between smaller and larger symbionts benefits the productivity of the symbiosis as a whole.
Keywords: Riftia pachyptila; cell differentiation; cell heterogeneity; host-microbe interaction; infectious disease; microbiology; sulfur-oxidizing symbiont; symbiosis.
© 2021, Hinzke et al.
Conflict of interest statement
TH, MK, MM, RS, CH, JP, PH, HF, SS, FB, UV, DB, TS, SM No competing interests declared
Figures








References
-
- Barabote RD, Johnson OL, Zetina E, San Francisco SK, Fralick JA, San Francisco MJ. Erwinia chrysanthemi tolC is involved in resistance to antimicrobial plant chemicals and is essential for phytopathogenesis. Journal of Bacteriology. 2003;185:5772–5778. doi: 10.1128/JB.185.19.5772-5778.2003. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

