PIN FORMED 2 Modulates the Transport of Arsenite in Arabidopsis thaliana
- PMID: 33404549
- PMCID: PMC7747963
- DOI: 10.1016/j.xplc.2019.100009
PIN FORMED 2 Modulates the Transport of Arsenite in Arabidopsis thaliana
Abstract
Arsenic contamination is a major environmental issue, as it may lead to serious health hazard. The reduced trivalent form of inorganic arsenic, arsenite, is in general more toxic to plants compared with the fully oxidized pentavalent arsenate. The uptake of arsenite in plants has been shown to be mediated through a large subfamily of plant aquaglyceroporins, nodulin 26-like intrinsic proteins (NIPs). However, the efflux mechanisms, as well as the mechanism of arsenite-induced root growth inhibition, remain poorly understood. Using molecular physiology, synchrotron imaging, and root transport assay approaches, we show that the cellular transport of trivalent arsenicals in Arabidopsis thaliana is strongly modulated by PIN FORMED 2 (PIN2) auxin efflux transporter. Root transport assay using radioactive arsenite, X-ray fluorescence imaging (XFI) coupled with X-ray absorption spectroscopy (XAS), and inductively coupled plasma mass spectrometry analysis revealed that pin2 plants accumulate higher concentrations of arsenite in roots compared with the wild-type. At the cellular level, arsenite specifically targets intracellular sorting of PIN2 and thereby alters the cellular auxin homeostasis. Consistently, loss of PIN2 function results in arsenite hypersensitivity in roots. XFI coupled with XAS further revealed that loss of PIN2 function results in specific accumulation of arsenical species, but not the other metals such as iron, zinc, or calcium in the root tip. Collectively, these results suggest that PIN2 likely functions as an arsenite efflux transporter for the distribution of arsenical species in planta.
Keywords: PIN2; arsenite; auxin; trafficking; transport.
© 2019 The Author(s).
Figures
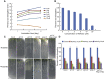

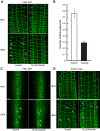
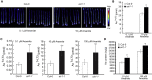

Comment in
-
A New Year's spotlight on two years of publication.Plant Commun. 2021 Dec 29;3(1):100274. doi: 10.1016/j.xplc.2021.100274. eCollection 2022 Jan 10. Plant Commun. 2021. PMID: 35059635 Free PMC article. No abstract available.
References
-
- Abas L., Benjamins R., Malenica N., Paciorek T.T., Wiřniewska J., Moulinier-Anzola J.C., Sieberer T., Friml J., Luschnig C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 2006;8:249–256. - PubMed
-
- Aida M., Vernoux T., Furutani M., Traas J., Tasaka M. Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development. 2002;129:3965–3974. - PubMed
-
- Ashraf M.A., Rahman A. Cold stress response in Arabidopsis thaliana is mediated by GNOM ARF-GEF. Plant J. 2019;97:500–516. - PubMed
-
- Barbosa I.C.R., Hammes U.Z., Schwechheimer C. Activation and polarity control of PIN-FORMED auxin transporters by phosphorylation. Trends Plant Sci. 2018;23:523–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

