Efficient photorelease of carbon monoxide from a luminescent tricarbonyl rhenium(I) complex incorporating pyridyl-1,2,4-triazole and phosphine ligands
- PMID: 33404562
- PMCID: PMC8177739
- DOI: 10.1039/d0dt03577g
Efficient photorelease of carbon monoxide from a luminescent tricarbonyl rhenium(I) complex incorporating pyridyl-1,2,4-triazole and phosphine ligands
Abstract
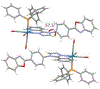
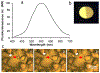
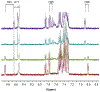
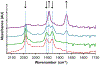
Precise control over the production of carbon monoxide (CO) is essential to exploit the therapeutic potential of this molecule. The development of photoactive CO-releasing molecules (PhotoCORMs) is therefore a promising route for future clinical applications. Herein, a tricarbonyl-rhenium(i) complex (1-TPP), which incorporates a phosphine moiety as ancilliary ligand for boosting the photochemical reactivity, and a pyridyltriazole bidentate ligand with appended 2-phenylbenzoxazole moiety for the purpose of photoluminescence, was synthesized and characterized from a chemical and crystallographic point of view. Upon irradiation in the near-UV range, complex 1-TPP underwent fast photoreaction, which was monitored through changes of the UV-vis absorption and phosphorescence spectra. The photoproducts (i.e. the dicarbonyl solvento complex 2 and one CO molecule) were identified using FTIR, 1H NMR and HRMS. The results were interpreted on the basis of DFT/TD-DFT calculations. The effective photochemical release of CO associated with clear optical variations (the emitted light passed from green to orange-red) could make 1-TPP the prototype of new photochemically-active agents, potentially useful for integration in photoCORM materials.
Conflict of interest statement
CONFLICTS OF INTEREST
There are no conflicts to declare.
Figures





Similar articles
-
A luminescent and biocompatible photoCORM.J Am Chem Soc. 2012 Nov 7;134(44):18197-200. doi: 10.1021/ja3084434. Epub 2012 Oct 23. J Am Chem Soc. 2012. PMID: 23077984
-
Phenyl-pyta-tricarbonylrhenium(I) complexes: combining a simplified structure and steric hindrance to modulate the photoluminescence properties.Dalton Trans. 2021 Oct 12;50(39):13686-13698. doi: 10.1039/d1dt02161c. Dalton Trans. 2021. PMID: 34523629
-
Luminescence modulations of rhenium tricarbonyl complexes induced by structural variations.Inorg Chem. 2014 Jun 16;53(12):6204-23. doi: 10.1021/ic5007007. Epub 2014 Jun 6. Inorg Chem. 2014. PMID: 24905983
-
Recent development of luminescent rhenium(i) tricarbonyl polypyridine complexes as cellular imaging reagents, anticancer drugs, and antibacterial agents.Dalton Trans. 2017 Dec 21;46(47):16357-16380. doi: 10.1039/c7dt03465b. Epub 2017 Nov 7. Dalton Trans. 2017. PMID: 29110007 Review.
-
Anticancer and Antibiotic Rhenium Tri- and Dicarbonyl Complexes: Current Research and Future Perspectives.Molecules. 2022 Jan 15;27(2):539. doi: 10.3390/molecules27020539. Molecules. 2022. PMID: 35056856 Free PMC article. Review.
Cited by
-
The Effect of Monodentate Co-Ligands on the Properties of Pt(II) Complexes Bearing a Tridentate C^N*N-Luminophore.Molecules. 2023 Nov 29;28(23):7834. doi: 10.3390/molecules28237834. Molecules. 2023. PMID: 38067570 Free PMC article.
-
Scope of tetrazolo[1,5-a]quinoxalines in CuAAC reactions for the synthesis of triazoloquinoxalines, imidazoloquinoxalines, and rhenium complexes thereof.Beilstein J Org Chem. 2022 Aug 24;18:1088-1099. doi: 10.3762/bjoc.18.111. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 36105720 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

