Progression of Behavioral Disturbances and Neuropsychiatric Symptoms in Patients With Genetic Frontotemporal Dementia
- PMID: 33404617
- PMCID: PMC7788468
- DOI: 10.1001/jamanetworkopen.2020.30194
Progression of Behavioral Disturbances and Neuropsychiatric Symptoms in Patients With Genetic Frontotemporal Dementia
Erratum in
-
Error in Article Information.JAMA Netw Open. 2021 Mar 1;4(3):e217664. doi: 10.1001/jamanetworkopen.2021.7664. JAMA Netw Open. 2021. PMID: 33787918 Free PMC article. No abstract available.
Abstract
Importance: Behavioral disturbances are core features of frontotemporal dementia (FTD); however, symptom progression across the course of disease is not well characterized in genetic FTD.
Objective: To investigate behavioral symptom frequency and severity and their evolution and progression in different forms of genetic FTD.
Design, setting, and participants: This longitudinal cohort study, the international Genetic FTD Initiative (GENFI), was conducted from January 30, 2012, to May 31, 2019, at 23 multicenter specialist tertiary FTD research clinics in the United Kingdom, the Netherlands, Belgium, France, Spain, Portugal, Italy, Germany, Sweden, Finland, and Canada. Participants included a consecutive sample of 232 symptomatic FTD gene variation carriers comprising 115 with variations in C9orf72, 78 in GRN, and 39 in MAPT. A total of 101 carriers had at least 1 follow-up evaluation (for a total of 400 assessments). Gene variations were included only if considered pathogenetic.
Main outcomes and measures: Behavioral and neuropsychiatric symptoms were assessed across disease duration and evaluated from symptom onset. Hierarchical generalized linear mixed models were used to model behavioral and neuropsychiatric measures as a function of disease duration and variation.
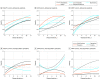
Results: Of 232 patients with FTD, 115 (49.6%) had a C9orf72 expansion (median [interquartile range (IQR)] age at evaluation, 64.3 [57.5-69.7] years; 72 men [62.6%]; 115 White patients [100%]), 78 (33.6%) had a GRN variant (median [IQR] age, 63.4 [58.3-68.8] years; 40 women [51.3%]; 77 White patients [98.7%]), and 39 (16.8%) had a MAPT variant (median [IQR] age, 56.3 [49.9-62.4] years; 25 men [64.1%]; 37 White patients [94.9%]). All core behavioral symptoms, including disinhibition, apathy, loss of empathy, perseverative behavior, and hyperorality, were highly expressed in all gene variant carriers (>50% patients), with apathy being one of the most common and severe symptoms throughout the disease course (51.7%-100% of patients). Patients with MAPT variants showed the highest frequency and severity of most behavioral symptoms, particularly disinhibition (79.3%-100% of patients) and compulsive behavior (64.3%-100% of patients), compared with C9orf72 carriers (51.7%-95.8% of patients with disinhibition and 34.5%-75.0% with compulsive behavior) and GRN carriers (38.2%-100% with disinhibition and 20.6%-100% with compulsive behavior). Alongside behavioral symptoms, neuropsychiatric symptoms were very frequently reported in patients with genetic FTD: anxiety and depression were most common in GRN carriers (23.8%-100% of patients) and MAPT carriers (26.1%-77.8% of patients); hallucinations, particularly auditory and visual, were most common in C9orf72 carriers (10.3%-54.5% of patients). Most behavioral and neuropsychiatric symptoms increased in the early-intermediate phases and plateaued in the late stages of disease, except for depression, which steadily declined in C9orf72 carriers, and depression and anxiety, which surged only in the late stages in GRN carriers.
Conclusions and relevance: This cohort study suggests that behavioral and neuropsychiatric disturbances differ between the common FTD gene variants and have different trajectories throughout the course of disease. These findings have crucial implications for counseling patients and caregivers and for the design of disease-modifying treatment trials in genetic FTD.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

