Does GEC1 Enhance Expression and Forward Trafficking of the Kappa Opioid Receptor (KOR) via Its Ability to Interact with NSF Directly?
- PMID: 33404775
- PMCID: PMC9126001
- DOI: 10.1007/164_2020_398
Does GEC1 Enhance Expression and Forward Trafficking of the Kappa Opioid Receptor (KOR) via Its Ability to Interact with NSF Directly?
Abstract
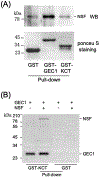
We reported previously that GEC1 (glandular epithelial cell 1), a member of microtubule-associated proteins (MAPs), interacted directly with the C-tail of KOR (KCT) and tubulin and enhanced cell surface expression of KOR in CHO cells by facilitating its trafficking along the export pathway. Two GEC1 analogs (GABARAP and GATE16) were also shown to increase KOR expression. In addition, to understand the underlying mechanism, we demonstrated that N-ethylmaleimide-sensitive factor (NSF), an essential component for membrane fusion, co-immunoprecipitated with GEC1 from brain extracts. In this study, using pull-down techniques, we have found that (1) GEC1 interacts with NSF directly and prefers the ADP-bound NSF to the ATP-bound NSF; (2) D1 and/or D2 domain(s) of NSF interact with GEC1, but the N domain of NSF does not; (3) NSF does not interact with KCT directly, but forms a protein complex with KCT via GEC1; (4) NSF and/or α-SNAP do not affect KCT-GEC1 interaction. Thus, GEC1 (vs the α-SNAP/SNAREs complex) binds to NSF in distinctive ways in terms of the ADP- or ATP-bound form and domains of NSF involved. In conclusion, GEC1 may, via its direct interactions with KOR, NSF, and tubulin, enhance trafficking and fusion of KOR-containing vesicles selectively along the export pathway, which leads to increase in surface expression of KOR. GABARAP and GATE16 may enhance KOR expression in a similar way.
Keywords: GABARAP; GATE16; GEC1; Kappa opioid receptor; NSF; Tubulin.
© 2020. The Author(s), under exclusive license to Springer Nature Switzerland AG.
Conflict of interest statement
Declarations of interest:
none
Figures


Similar articles
-
GEC1 interacts with the kappa opioid receptor and enhances expression of the receptor.J Biol Chem. 2006 Mar 24;281(12):7983-93. doi: 10.1074/jbc.M509805200. Epub 2006 Jan 23. J Biol Chem. 2006. PMID: 16431922
-
Effects of C-terminal modifications of GEC1 protein and gamma-aminobutyric acid type A (GABA(A)) receptor-associated protein (GABARAP), two microtubule-associated proteins, on kappa opioid receptor expression.J Biol Chem. 2011 Apr 29;286(17):15106-15. doi: 10.1074/jbc.M111.230896. Epub 2011 Mar 9. J Biol Chem. 2011. PMID: 21388957 Free PMC article.
-
GEC1-kappa opioid receptor binding involves hydrophobic interactions: GEC1 has chaperone-like effect.J Biol Chem. 2009 Jan 16;284(3):1673-85. doi: 10.1074/jbc.M808303200. Epub 2008 Nov 11. J Biol Chem. 2009. PMID: 19001416 Free PMC article.
-
Cellular functions of NSF: not just SNAPs and SNAREs.FEBS Lett. 2007 May 22;581(11):2140-9. doi: 10.1016/j.febslet.2007.03.032. Epub 2007 Mar 21. FEBS Lett. 2007. PMID: 17397838 Free PMC article. Review.
-
Requirements for the catalytic cycle of the N-ethylmaleimide-Sensitive Factor (NSF).Biochim Biophys Acta. 2012 Jan;1823(1):159-71. doi: 10.1016/j.bbamcr.2011.06.003. Epub 2011 Jun 13. Biochim Biophys Acta. 2012. PMID: 21689688 Free PMC article. Review.
References
-
- Chen C, Li JG, Chen Y, Huang P, Wang Y, Liu-Chen LY (2006) GEC1 interacts with the kappa opioid receptor and enhances expression of the receptor. The Journal of biological chemistry 281: 7983–93. - PubMed
-
- Chen C, Wang Y, Huang P, Liu-Chen LY (2011) Effects of C-terminal modifications of GEC1 protein and gamma-aminobutyric acid type A (GABA(A)) receptor-associated protein (GABARAP), two microtubule-associated proteins, on kappa opioid receptor expression. The Journal of biological chemistry 286: 15106–15. - PMC - PubMed
-
- Chen L, Wang H, Vicini S, Olsen RW (2000) The gamma-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP) promotes GABAA receptor clustering and modulates the channel kinetics. Proceedings of the National Academy of Sciences of the United States of America 97: 11557–62. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

