Capillary Leukocytes, Microaggregates, and the Response to Hypoxemia in the Microcirculation of Coronavirus Disease 2019 Patients
- PMID: 33405410
- PMCID: PMC7963442
- DOI: 10.1097/CCM.0000000000004862
Capillary Leukocytes, Microaggregates, and the Response to Hypoxemia in the Microcirculation of Coronavirus Disease 2019 Patients
Abstract
Objectives: In this study, we hypothesized that coronavirus disease 2019 patients exhibit sublingual microcirculatory alterations caused by inflammation, coagulopathy, and hypoxemia.
Design: Multicenter case-controlled study.
Setting: Two ICUs in The Netherlands and one in Switzerland.
Patients: Thirty-four critically ill coronavirus disease 2019 patients were compared with 33 healthy volunteers.
Interventions: None.
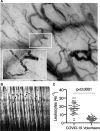
Measurements and main results: The microcirculatory parameters quantified included total vessel density (mm × mm-2), functional capillary density (mm × mm-2), proportion of perfused vessels (%), capillary hematocrit (%), the ratio of capillary hematocrit to systemic hematocrit, and capillary RBC velocity (μm × s-1). The number of leukocytes in capillary-postcapillary venule units per 4-second image sequence (4 s-1) and capillary RBC microaggregates (4 s-1) was measured. In comparison with healthy volunteers, the microcirculation of coronavirus disease 2019 patients showed increases in total vessel density (22.8 ± sd 5.1 vs 19.9 ± 3.3; p < 0.0001) and functional capillary density (22.2 ± 4.8 vs 18.8 ± 3.1; p < 0.002), proportion of perfused vessel (97.6 ± 2.1 vs 94.6 ± 6.5; p < 0.01), RBC velocity (362 ± 48 vs 306 ± 53; p < 0.0001), capillary hematocrit (5.3 ± 1.3 vs 4.7 ± 0.8; p < 0.01), and capillary-hematocrit-to-systemic-hematocrit ratio (0.18 ± 0.0 vs 0.11 ± 0.0; p < 0.0001). These effects were present in coronavirus disease 2019 patients with Sequential Organ Failure Assessment scores less than 10 but not in patients with Sequential Organ Failure Assessment scores greater than or equal to 10. The numbers of leukocytes (17.6 ± 6.7 vs 5.2 ± 2.3; p < 0.0001) and RBC microaggregates (0.90 ± 1.12 vs 0.06 ± 0.24; p < 0.0001) was higher in the microcirculation of the coronavirus disease 2019 patients. Receiver-operating-characteristics analysis of the microcirculatory parameters identified the number of microcirculatory leukocytes and the capillary-hematocrit-to-systemic-hematocrit ratio as the most sensitive parameters distinguishing coronavirus disease 2019 patients from healthy volunteers.
Conclusions: The response of the microcirculation to coronavirus disease 2019-induced hypoxemia seems to be to increase its oxygen-extraction capacity by increasing RBC availability. Inflammation and hypercoagulation are apparent in the microcirculation by increased numbers of leukocytes and RBC microaggregates.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and Wolters Kluwer Health, Inc.
Conflict of interest statement
Dr. Ince has received honoraria and independent research grants from Fresenius-Kabi, Bad Homburg, Germany; La Jolla Pharmaceutical, La Jolla, CA; and Cytosorbents Monmouth, NJ. He has developed sidestream dark field imaging, which is the handheld video microscope and is listed as the inventor on related patents commercialized by MicroVision Medical (MVM) under a license from the Academic Medical Center. He receives no royalties or benefits from this license. He has been a consultant for MVM in the past but has not been involved with this company for more than 5 years now and holds no shares of stock. Braedius Medical, a company owned by a relative of Dr. Ince, has developed and designed the incident dark field device used in this study. Dr. Ince has no financial relationship with Braedius Medical of any sort and has never owned shares, or received consultancy or speaker fees from Braedius Medical. The MicroTools software is being developed by Dr. Hilty and owned by Active Medical BV Leiden, The Netherlands, of which Drs. Ince and Hilty are shareholders. Active Medical runs an Internet site called microcirculationacademy.org, which offers educational courses and services related to clinical microcirculation. Dr. Ince’s institution received funding from La Jolla Pharmaceuticals and Cytosorbents Monmouth, and he received funding from Fresenius-Kabi. Drs. Ince and Hilty disclosed that the MicroTools software that was used for analysis of the images in the current study is owned by Active Medical, of which Drs. Ince and Hilty own shares. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
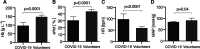


References
-
- Joffre J, Hellman J, Ince C, et al. . Endothelial responses in sepsis. Am J Respir Crit Care Med. 2020; 202:361–370 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

