Proton-Coupled Electron Transfer Guidelines, Fair and Square
- PMID: 33405896
- PMCID: PMC7880575
- DOI: 10.1021/jacs.0c09106
Proton-Coupled Electron Transfer Guidelines, Fair and Square
Abstract
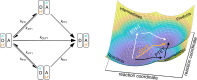
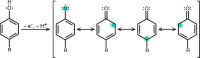
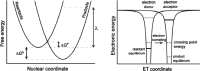
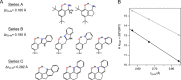
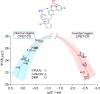
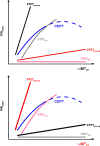
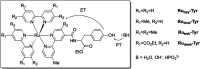
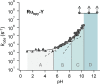
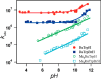
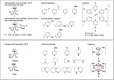
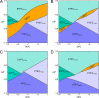
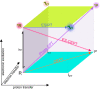
Proton-coupled electron transfer (PCET) reactions are fundamental to energy transformation reactions in natural and artificial systems and are increasingly recognized in areas such as catalysis and synthetic chemistry. The interdependence of proton and electron transfer brings a mechanistic richness of reactivity, including various sequential and concerted mechanisms. Delineating between different PCET mechanisms and understanding why a particular mechanism dominates are crucial for the design and optimization of reactions that use PCET. This Perspective provides practical guidelines for how to discern between sequential and concerted mechanisms based on interpretations of thermodynamic data with temperature-, pressure-, and isotope-dependent kinetics. We present new PCET-zone diagrams that show how a mechanism can switch or even be eliminated by varying the thermodynamic (ΔGPT° and ΔGET°) and coupling strengths for a PCET system. We discuss the appropriateness of asynchronous concerted PCET to rationalize observations in organic reactions, and the distinction between hydrogen atom transfer and other concerted PCET reactions. Contemporary issues and future prospects in PCET research are discussed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Trefil J.; Morowitz H. J.; Smith E. The Origin of Life: A case is made for the descent of electrons. Am. Sci. 2009, 97 (3), 206–213. 10.1511/2009.78.206. - DOI
-
- Peper J. L.; Mayer J. M. Manifesto on the Thermochemistry of Nanoscale Redox Reactions for Energy Conversion. ACS Ener. Lett. 2019, 4 (4), 866–872. 10.1021/acsenergylett.9b00019. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

