Clinical Implications of Minimal Residual Disease Detection in Infants With KMT2A-Rearranged Acute Lymphoblastic Leukemia Treated on the Interfant-06 Protocol
- PMID: 33405950
- PMCID: PMC8196086
- DOI: 10.1200/JCO.20.02333
Clinical Implications of Minimal Residual Disease Detection in Infants With KMT2A-Rearranged Acute Lymphoblastic Leukemia Treated on the Interfant-06 Protocol
Erratum in
-
Erratum: Clinical Implications of Minimal Residual Disease Detection in Infants With KMT2A-Rearranged Acute Lymphoblastic Leukemia Treated on the Interfant-06 Protocol.J Clin Oncol. 2023 Oct 20;41(30):4825. doi: 10.1200/JCO.23.01844. Epub 2023 Sep 12. J Clin Oncol. 2023. PMID: 37699172 Free PMC article. No abstract available.
Abstract
Purpose: Infant acute lymphoblastic leukemia (ALL) is characterized by a high incidence of KMT2A gene rearrangements and poor outcome. We evaluated the value of minimal residual disease (MRD) in infants with KMT2A-rearranged ALL treated within the Interfant-06 protocol, which compared lymphoid-style consolidation (protocol IB) versus myeloid-style consolidation (araC, daunorubicin, etoposide/mitoxantrone, araC, etoposide).
Materials and methods: MRD was measured in 249 infants by DNA-based polymerase chain reaction of rearranged KMT2A, immunoglobulin, and/or T-cell receptor genes, at the end of induction (EOI) and end of consolidation (EOC). MRD results were classified as negative, intermediate (< 5 × 10-4), and high (≥ 5 × 10-4).
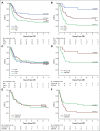
Results: EOI MRD levels predicted outcome with 6-year disease-free survival (DFS) of 60.2% (95% CI, 43.2 to 73.6), 45.0% (95% CI, 28.3 to 53.1), and 33.8% (95% CI, 23.8 to 44.1) for infants with negative, intermediate, and high EOI MRD levels, respectively (P = .0039). EOC MRD levels were also predictive of outcome, with 6-year DFS of 68.2% (95% CI, 55.2 to 78.1), 40.1% (95% CI, 28.1 to 51.9), and 11.9% (95% CI, 2.6 to 29.1) for infants with negative, intermediate, and high EOC MRD levels, respectively (P < .0001). Analysis of EOI MRD according to the type of consolidation treatment showed that infants treated with lymphoid-style consolidation had 6-year DFS of 78.2% (95% CI, 51.4 to 91.3), 47.2% (95% CI, 33.0 to 60.1), and 23.2% (95% CI, 12.1 to 36.4) for negative, intermediate, and high MRD levels, respectively (P < .0001), while for myeloid-style-treated patients the corresponding figures were 45.0% (95% CI, 23.9 to 64.1), 41.3% (95% CI, 23.2 to 58.5), and 45.9% (95% CI, 29.4 to 60.9).
Conclusion: This study provides support for the idea that induction therapy selects patients for subsequent therapy; infants with high EOI MRD may benefit from AML-like consolidation (DFS 45.9% v 23.2%), whereas patients with low EOI MRD may benefit from ALL-like consolidation (DFS 78.2% v 45.0%). Patients with positive EOC MRD had dismal outcomes. These findings will be used for treatment interventions in the next Interfant protocol.
Trial registration: ClinicalTrials.gov NCT00550992.
Figures

References
-
- Pieters R Schrappe M De Lorenzo P, et al. : A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): An observational study and a multicentre randomised trial. Lancet 370:240-250, 2007 - PubMed
-
- Pieters R De Lorenzo P Ancliffe P, et al. : Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the interfant-06 protocol: Results from an international phase III randomized study. J Clin Oncol 37:2246-2256, 2019 - PubMed
-
- Van Dongen JJ Seriu T Panzer-Grümayer ER, et al. : Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet 352:1731-1738, 1998 - PubMed
-
- Faderl S Kantarjian HM Talpaz M, et al. : Clinical significance of minimal residual disease in leukemia. Int J Oncol 17:1277-1287, 2000 - PubMed
-
- Pieters R de Groot-Kruseman H Van der Velden V, et al. : Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: Study ALL10 from the Dutch Childhood Oncology Group. J Clin Oncol 34:2591-2601, 2016 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

