Outcomes by Clinical and Molecular Features in Children With Medulloblastoma Treated With Risk-Adapted Therapy: Results of an International Phase III Trial (SJMB03)
- PMID: 33405951
- PMCID: PMC10166353
- DOI: 10.1200/JCO.20.01372
Outcomes by Clinical and Molecular Features in Children With Medulloblastoma Treated With Risk-Adapted Therapy: Results of an International Phase III Trial (SJMB03)
Abstract
Purpose: SJMB03 (ClinicalTrials.gov identifier: NCT00085202) was a phase III risk-adapted trial that aimed to determine the frequency and clinical significance of biological variants and genetic alterations in medulloblastoma.
Patients and methods: Patients 3-21 years old were stratified into average-risk and high-risk treatment groups based on metastatic status and extent of resection. Medulloblastomas were molecularly classified into subgroups (Wingless [WNT], Sonic Hedgehog [SHH], group 3, and group 4) and subtypes based on DNA methylation profiles and overlaid with gene mutations from next-generation sequencing. Coprimary study end points were (1) to assess the relationship between ERBB2 protein expression in tumors and progression-free survival (PFS), and (2) to estimate the frequency of mutations associated with WNT and SHH tumors. Clinical and molecular risk factors were evaluated, and the most robust were used to model new risk-classification categories.
Results: Three hundred thirty eligible patients with medulloblastoma were enrolled. Five-year PFS was 83.2% (95% CI, 78.4 to 88.2) for average-risk patients (n = 227) and 58.7% (95% CI, 49.8 to 69.1) for high-risk patients (n = 103). No association was found between ERBB2 status and PFS in the overall cohort (P = .74) or when patients were stratified by clinical risk (P = .71). Mutations in CTNNB1 (96%), DDX3X (37%), and SMARCA4 (24%) were most common in WNT tumors and PTCH1 (38%), TP53 (21%), and DDX3X (19%) in SHH tumors. Methylome profiling classified 53 WNT (17.4%), 48 SHH (15.7%), 65 group 3 (21.3%), and 139 group 4 (45.6%) tumors. A comprehensive clinicomolecular risk factor analysis identified three low-risk groups (WNT, low-risk SHH, and low-risk combined groups 3 and 4) with excellent (5-year PFS > 90%) and two very high-risk groups (high-risk SHH and high-risk combined groups 3 and 4) with poor survival (5-year PFS < 60%).
Conclusion: These results establish a new risk stratification for future medulloblastoma trials.
Conflict of interest statement
Outcomes by Clinical and Molecular Features in Children With Medulloblastoma Treated With Risk-Adapted Therapy: Results of an International Phase III Trial (SJMB03)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Amar Gajjar
Giles W. Robinson
Thomas E. Merchant
Eric Bouffet
Jordan R. Hansford
Geoffrey McCowage
Michael J. Fisher
Alberto Broniscer
Frederick Boop
Stefan M. Pfister
Richard J. Gilbertson
Arzu Onar-Thomas
David W. Ellison
No other potential conflicts of interest were reported.
Figures
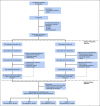
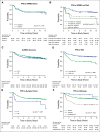
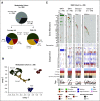
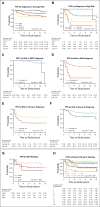
Comment in
-
Medulloblastoma: prognostic subtypes revealed.Nat Rev Clin Oncol. 2021 Mar;18(3):131. doi: 10.1038/s41571-021-00478-0. Nat Rev Clin Oncol. 2021. PMID: 33510481 No abstract available.
References
-
- Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. - PubMed
-
- von Bueren AO, Kortmann RD, von Hoff K, et al. Treatment of children and adolescents with metastatic medulloblastoma and prognostic relevance of clinical and biologic parameters. J Clin Oncol. 2016;34:4151–4160. - PubMed
-
- Lannering B, Rutkowski S, Doz F, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: Results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol. 2012;30:3187–3193. - PubMed
-
- Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

