Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults
- PMID: 33406353
- PMCID: PMC7793608
- DOI: 10.1056/NEJMoa2033700
Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults
Abstract
Background: Therapies to interrupt the progression of early coronavirus disease 2019 (Covid-19) remain elusive. Among them, convalescent plasma administered to hospitalized patients has been unsuccessful, perhaps because antibodies should be administered earlier in the course of illness.
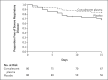
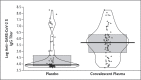
Methods: We conducted a randomized, double-blind, placebo-controlled trial of convalescent plasma with high IgG titers against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in older adult patients within 72 hours after the onset of mild Covid-19 symptoms. The primary end point was severe respiratory disease, defined as a respiratory rate of 30 breaths per minute or more, an oxygen saturation of less than 93% while the patient was breathing ambient air, or both. The trial was stopped early at 76% of its projected sample size because cases of Covid-19 in the trial region decreased considerably and steady enrollment of trial patients became virtually impossible.
Results: A total of 160 patients underwent randomization. In the intention-to-treat population, severe respiratory disease developed in 13 of 80 patients (16%) who received convalescent plasma and 25 of 80 patients (31%) who received placebo (relative risk, 0.52; 95% confidence interval [CI], 0.29 to 0.94; P = 0.03), with a relative risk reduction of 48%. A modified intention-to-treat analysis that excluded 6 patients who had a primary end-point event before infusion of convalescent plasma or placebo showed a larger effect size (relative risk, 0.40; 95% CI, 0.20 to 0.81). No solicited adverse events were observed.
Conclusions: Early administration of high-titer convalescent plasma against SARS-CoV-2 to mildly ill infected older adults reduced the progression of Covid-19. (Funded by the Bill and Melinda Gates Foundation and the Fundación INFANT Pandemic Fund; Dirección de Sangre y Medicina Transfusional del Ministerio de Salud number, PAEPCC19, Plataforma de Registro Informatizado de Investigaciones en Salud number, 1421, and ClinicalTrials.gov number, NCT04479163.).
Copyright © 2021 Massachusetts Medical Society.
Figures
Comment in
-
(A Little) Clarity on Convalescent Plasma for Covid-19.N Engl J Med. 2021 Feb 18;384(7):666-668. doi: 10.1056/NEJMe2035678. Epub 2021 Jan 13. N Engl J Med. 2021. PMID: 33440086 Free PMC article. No abstract available.
-
Plasma Therapy to Prevent Severe Covid-19 in Older Adults.N Engl J Med. 2021 Jun 24;384(25):e104. doi: 10.1056/NEJMc2104747. Epub 2021 May 12. N Engl J Med. 2021. PMID: 33979509 No abstract available.
-
Plasma Therapy to Prevent Severe Covid-19 in Older Adults.N Engl J Med. 2021 Jun 24;384(25):e104. doi: 10.1056/NEJMc2104747. Epub 2021 May 12. N Engl J Med. 2021. PMID: 33979510 No abstract available.
-
COVID-19 convalescent plasma donor recruitment experience from the perspective of a hospital transfusion medicine service.Transfusion. 2021 Jul;61(7):2213-2215. doi: 10.1111/trf.16448. Epub 2021 Jun 13. Transfusion. 2021. PMID: 33990952 Free PMC article. No abstract available.
-
Rekonvaleszenten-Plasma mildert COVID-19-Verlauf bei Älteren.MMW Fortschr Med. 2021 Jun;163(11):28. doi: 10.1007/s15006-021-0068-4. MMW Fortschr Med. 2021. PMID: 34086221 Free PMC article. German. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
