NLRC4 gene silencing-dependent blockade of NOD-like receptor pathway inhibits inflammation, reduces proliferation and increases apoptosis of dendritic cells in mice with septic shock
- PMID: 33406504
- PMCID: PMC7835030
- DOI: 10.18632/aging.202379
NLRC4 gene silencing-dependent blockade of NOD-like receptor pathway inhibits inflammation, reduces proliferation and increases apoptosis of dendritic cells in mice with septic shock
Abstract
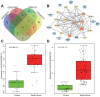
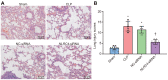
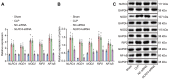
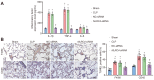
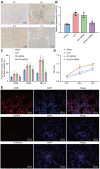
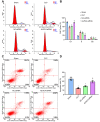
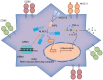
Septic shock is one of the most significant health concerns across the world, involving hypo-perfusion and defects in tissue energy. The current study investigates the role of NLR family CARD domain containing protein 4 (NLRC4) in septic shock-induced inflammatory reactions, lung tissue injuries, and dendritic cell (DC) apoptosis. Septic shock mice models were established by modified cecal ligation and puncture and injected with retroviral vector expressing siRNA-NLRC4. DCs were then isolated and transfected with siRNA-NLRC4. The degree of lung tissue injury, cell cycle distribution, cell apoptosis and cell viability of DCs were assessed. NLRC4 was found to be expressed at high levels in mice with septic shock. NLRC4 silencing inhibited the activation of the NOD-like receptor (NLR) pathway as evidenced by the decreased levels of NOD1, NOD2, RIP2, and NF-κB. In addition, NLRC4 silencing reduced the inflammatory reaction as attributed by reduced levels of IL-1β, TNF-α and IL-6. Suppressed NLRC4 levels inhibited cell viability and promoted cell apoptosis evidenced by inhibited induction of DC surface markers (CD80, CD86, and MHC II), along with alleviated lung tissue injury. In conclusion, NLRC4 silencing ameliorates lung injury and inflammation induced by septic shock by negatively regulating the NLR pathway.
Keywords: NLRC4; NOD-like receptor pathway; immune response; inflammatory reaction; septic shock.
Conflict of interest statement
Figures
References
-
- Meyer NJ, Ferguson JF, Feng R, Wang F, Patel PN, Li M, Xue C, Qu L, Liu Y, Boyd JH, Russell JA, Christie JD, Walley KR, Reilly MP. A functional synonymous coding variant in the IL1RN gene is associated with survival in septic shock. Am J Respir Crit Care Med. 2014; 190:656–64. 10.1164/rccm.201403-0586OC - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

