The Evolutionary Landscape of Treatment for BRAFV600E Mutant Metastatic Colorectal Cancer
- PMID: 33406649
- PMCID: PMC7795863
- DOI: 10.3390/cancers13010137
The Evolutionary Landscape of Treatment for BRAFV600E Mutant Metastatic Colorectal Cancer
Abstract
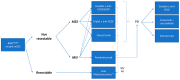
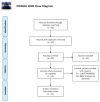
The BRAFV600E mutation is found in 8-10% of metastatic colorectal cancer (mCRC) patients and it is recognized as a poor prognostic factor with a median overall survival inferior to 20 months. At present, besides immune checkpoint inhibitors (CPIs) for those tumors with concomitant MSI-H status, recommended treatment options include cytotoxic chemotherapy + anti-VEGF in the first line setting, and a combination of EGFR and a BRAF inhibitor (cetuximab plus encorafenib) in second line. However, even with the latter targeted approach, acquired resistance limits the possibility of more than an incremental benefit and survival is still dismal. In this review, we discuss current treatment options for this subset of patients and perform a systematic review of ongoing clinical trials. Overall, we identified six emerging strategies: targeting MAPK pathway (monotherapy or combinations), targeting MAPK pathway combined with cytotoxic agents, intensive cytotoxic regimen combinations, targeted agents combined with CPIs, oxidative stress induction, and cytotoxic agents combined with antiangiogenic drugs and CPIs. In the future, the integration of new therapeutic strategies targeting key players in the BRAFV600E oncogenic pathways with current treatment approach based on cytotoxic chemotherapy and surgery is likely to redefine the treatment landscape of these CRC patients.
Keywords: BRAF; FOLFOXIRI; colon cancer; immune checkpoint inhibitors; targeted agents.
Conflict of interest statement
Salvatore Siena is advisory board member for Amgen, Bayer, BMS, CheckmAb, Clovis, Daiichi-Sankyo, Merck, Roche-Genentech, and Seattle Genetics. Andrea Sartore-Bianchi is advisory board member for Amgen, Bayer, Sanofi and Servier. The other authors declare no competing interests.
Figures

References
-
- NCCN Clinical Practice Guidelines in Oncology. [(accessed on 21 March 2020)]; Available online: https://www.nccn.org/professionals/physician_gls/default.aspx.
-
- Van Cutsem E., Cervantes A., Adam R., Sobrero A., Van Krieken J.H., Aderka D., Aranda Aguilar E., Bardelli A., Benson A., Bodoky G., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. - DOI - PubMed
-
- Yoshino T., Arnold D., Taniguchi H., Pentheroudakis G., Yamazaki K., Xu R.-H., Kim T.W., Ismail F., Tan I.B., Yeh K.-H., et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 2018;29:44–70. doi: 10.1093/annonc/mdx738. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

