Impact of Environmental Factors on Stilbene Biosynthesis
- PMID: 33406721
- PMCID: PMC7823792
- DOI: 10.3390/plants10010090
Impact of Environmental Factors on Stilbene Biosynthesis
Abstract
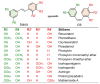
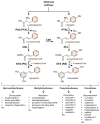
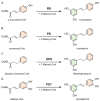
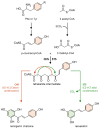
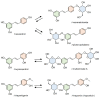
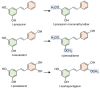
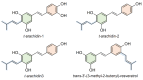
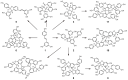
Stilbenes are a small family of polyphenolic secondary metabolites that can be found in several distantly related plant species. These compounds act as phytoalexins, playing a crucial role in plant defense against phytopathogens, as well as being involved in the adaptation of plants to abiotic environmental factors. Among stilbenes, trans-resveratrol is certainly the most popular and extensively studied for its health properties. In recent years, an increasing number of stilbene compounds were subjected to investigations concerning their bioactivity. This review presents the most updated knowledge of the stilbene biosynthetic pathway, also focusing on the role of several environmental factors in eliciting stilbenes biosynthesis. The effects of ultraviolet radiation, visible light, ultrasonication, mechanical stress, salt stress, drought, temperature, ozone, and biotic stress are reviewed in the context of enhancing stilbene biosynthesis, both in planta and in plant cell and organ cultures. This knowledge may shed some light on stilbene biological roles and represents a useful tool to increase the accumulation of these valuable compounds.
Keywords: biosynthetic pathway; environmental factors; phenylpropanoid pathway; phytoalexins; pinosylvin synthase; polyphenols; resveratrol synthase; secondary metabolites; stilbene biosynthesis; stilbene synthase; stilbenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Medicinal chemistry perspective on the structure-activity relationship of stilbene derivatives.RSC Adv. 2024 Jun 20;14(28):19823-19879. doi: 10.1039/d4ra02867h. eCollection 2024 Jun 18. RSC Adv. 2024. PMID: 38903666 Free PMC article. Review.
-
Stilbene accumulation and expression of stilbene biosynthesis pathway genes in wild grapevine Vitis amurensis Rupr.Planta. 2017 Jan;245(1):151-159. doi: 10.1007/s00425-016-2598-z. Epub 2016 Sep 29. Planta. 2017. PMID: 27686467
-
Regulation of stilbene biosynthesis in plants.Planta. 2017 Oct;246(4):597-623. doi: 10.1007/s00425-017-2730-8. Epub 2017 Jul 6. Planta. 2017. PMID: 28685295 Review.
-
Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants.Biofactors. 2010 Sep-Oct;36(5):331-41. doi: 10.1002/biof.108. Biofactors. 2010. PMID: 20726013 Review.
-
Induction of stilbene phytoalexins in grapevine (Vitis vinifera) and transgenic stilbene synthase-apple plants (Malus domestica) by a culture filtrate of Aureobasidium pullulans.Plant Physiol Biochem. 2013 Nov;72:62-71. doi: 10.1016/j.plaphy.2013.03.011. Epub 2013 Mar 23. Plant Physiol Biochem. 2013. PMID: 23578977
Cited by
-
Evidence of an active role of resveratrol derivatives in the tolerance of wild grapevines (Vitis vinifera ssp. sylvestris) to salinity.J Plant Res. 2024 Mar;137(2):265-277. doi: 10.1007/s10265-023-01515-y. Epub 2023 Dec 27. J Plant Res. 2024. PMID: 38148429
-
Natural Compounds as Promising Adjuvant Agents in The Treatment of Gliomas.Int J Mol Sci. 2022 Mar 20;23(6):3360. doi: 10.3390/ijms23063360. Int J Mol Sci. 2022. PMID: 35328780 Free PMC article. Review.
-
Abscisic Acid and Chitosan Modulate Polyphenol Metabolism and Berry Qualities in the Domestic White-Colored Cultivar Savvatiano.Plants (Basel). 2022 Jun 22;11(13):1648. doi: 10.3390/plants11131648. Plants (Basel). 2022. PMID: 35807600 Free PMC article.
-
A comprehensive review of the transcriptomic and metabolic responses of grapevines to arbuscular mycorrhizal fungi.Planta. 2025 Jul 17;262(3):58. doi: 10.1007/s00425-025-04771-5. Planta. 2025. PMID: 40676374 Review.
-
New Insights into Dietary Pterostilbene: Sources, Metabolism, and Health Promotion Effects.Molecules. 2022 Sep 25;27(19):6316. doi: 10.3390/molecules27196316. Molecules. 2022. PMID: 36234852 Free PMC article. Review.
References
-
- El Khawand T., Courtois A., Valls J., Richard T., Krisa S. A review of dietary stilbenes: Sources and bioavailability. Phytochem. Rev. 2018;17:1007–1029. doi: 10.1007/s11101-018-9578-9. - DOI
-
- Chong J., Poutaraud A., Hugueney P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009;177:143–155. doi: 10.1016/j.plantsci.2009.05.012. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

