Inhibition of Enterovirus A71 by a Novel 2-Phenyl-Benzimidazole Derivative
- PMID: 33406781
- PMCID: PMC7823780
- DOI: 10.3390/v13010058
Inhibition of Enterovirus A71 by a Novel 2-Phenyl-Benzimidazole Derivative
Abstract
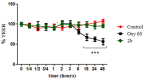
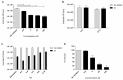
Enterovirus A71 (EV-A71) infection has emerged as a significant public health concern atthe global level. Epidemic events of EV-A71 have been reported worldwide, and this succession of outbreaks has heightened concern that EV-A71 may become a public health threat. In recent years, widespread A71 enterovirus also occurred in European countries. EV-A71 infection causes hand-foot-mouth disease (HFMD), herpangina, and fever. However, it can sometimes induce a variety of neurological complications, including encephalitis, aseptic meningitis, pulmonary edema, and acute flaccid paralysis. We identified new benzimidazole derivatives and described their in vitro cytotoxicity and broad-spectrum anti-enterovirus activity. Among them, derivative 2b resulted in interesting activity against EV-A71, and therefore it was selected for further investigations. Compound 2b proved to be able to protect cell monolayers from EV-A71-induced cytopathogenicity, with an EC50 of 3 µM. Moreover, Vero-76 cells resulted in being significantly protected from necrosis and apoptosis when treated with 2b at 20 and 80 µM. Compound 2b reduced viral adsorption to Vero-76 cells, and when evaluated in a time-of-addition assay, the derivative had the highest effect when added during the infection period. Moreover, derivative 2b reduced viral penetration into host cells. Besides, 2b did not affect intestinal monolayers permeability, showing no toxic effects. A detailed insight into the efficacy of compound 2b against EV-A71 showed a dose-dependent reduction in the viral titer, also at low concentrations. Mechanism of action investigations suggested that our derivative can inhibit viral endocytosis by reducing viral attachment to and penetration into host cells. Pharmacokinetic and toxicity predictions validated compound 2b as a good candidate for further in vivo assays.
Keywords: EV-A71; TEER; antivirals; apoptosis assay; benzimidazole derivatives; neurological complications; penetration assay; timecourse.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

