Denosumab Discontinuation and the Rebound Phenomenon: A Narrative Review
- PMID: 33406802
- PMCID: PMC7796169
- DOI: 10.3390/jcm10010152
Denosumab Discontinuation and the Rebound Phenomenon: A Narrative Review
Abstract
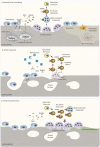
Denosumab is a potent antiresorptive agent that substantially increases bone mineral density and reduces fracture rates at all skeletal sites for as long as it is administered. However, its favorable skeletal effects reverse quickly upon its discontinuation, because of a vast increase of osteoclast number and activity, which leads to a subsequent profound increase of bone turnover above pre-treatment values, a phenomenon commonly described as "rebound phenomenon". More importantly, most patients experience rapid, profound bone loss due to this burst of bone resorption that may lead in a minority of these patients to occurrence of fractures, especially multiple vertebral fractures. Therefore, subsequent antiresorptive treatment is mandatory, although the optimal regimen is yet to be clarified. In the present review, we outline what is currently known regarding the negative effects of denosumab discontinuation on different aspects of bone status, the factors that may affect them, and strategies to prevent them.
Keywords: denosumab; discontinuation; fracture; osteoporosis; rebound; turnover.
Conflict of interest statement
A.D. Anastasilakis reports lecture fees from Amgen, UCB, Bianex, Eli-Lilly and ITF; P. Makras reports honoraria for lectures and research grants from Amgen and lecture fees from UCB, Glaxo, Lilly, Pfizer, Leo, Genesis, Elpen, Galenica, Takeda, and Bianex; M.P. Yavropoulou has received lecture fees from Eli-Lilly, Galenica S.A., Shire and UCB S.A.; G. Tabacco has no conflict of interest; A.M. Naciu reports lecture fees from Amgen; A. Palermo reports lecture fees from Amgen.
Figures
References
-
- Bone H.G., Wagman R.B., Brandi M.L., Brown J.P., Chapurlat R., Cummings S.R., Czerwiński E., Fahrleitner-Pammer A., Kendler D.L., Lippuner K., et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–523. doi: 10.1016/S2213-8587(17)30138-9. - DOI - PubMed
-
- Bone H.G., Bolognese M.A., Yuen C.K., Kendler D.L., Miller P.D., Yang Y.-C., Grazette L., Martin J.S., Gallagher J.C. Effects of Denosumab Treatment and Discontinuation on Bone Mineral Density and Bone Turnover Markers in Postmenopausal Women with Low Bone Mass. J. Clin. Endocrinol. Metab. 2011;96:972–980. doi: 10.1210/jc.2010-1502. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

