Association between response to triptans and response to erenumab: real-life data
- PMID: 33407070
- PMCID: PMC7789681
- DOI: 10.1186/s10194-020-01213-3
Association between response to triptans and response to erenumab: real-life data
Abstract
Background: Triptans and erenumab are both migraine-specific agents acting on the calcitonin gene-related peptide pathway. Therefore, response to triptans might be associated with response to erenumab.
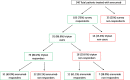
Main body: In our study, consecutive patients referring to the Headache Centers of the Abruzzo region from January 2019 to March 2020 and treated with erenumab were interviewed about past use and efficacy of triptans. Triptan users were classified as 'triptan responders' if they were headache-free 2 h after treating ≥3 migraine attacks with ≥1 triptan. We considered patients as 'erenumab responders', if they had a ≥ 50% mean reduction in monthly migraine days between the 4th and the 6th month from treatment start compared with baseline. Of 91 triptan users, 73 (80.2%) were triptan responders and 58 (63.7%) were erenumab responders. The odds ratio of being erenumab responder was 3.64 (95% CI, 1.25-10.64) for triptan users as compared to non-users. (P = 0.014). Besides, starting erenumab improved triptan response in both erenumab responders and non-responders.
Conclusions: Our data of an association between response to triptans and response to erenumab can be useful for patient advice and to improve the understanding of migraine pathophysiology and treatment.
Keywords: CGRP; Erenumab; Migraine treatment; Triptans.
Conflict of interest statement
RO declares financial and non-financial relationships with Eli Lilly and Novartis, non-financial relationships with Allergan, and Teva; SS had a financial relationship (lecturer or member of advisory board) with Abbott, Allergan, Novartis, Teva, and Eli Lilly; GA has received funds for congress participation from Innovet Italia Srl, Epitech Group and Lusofarmaco; MAG received funds for congress participation from IBSA; AC, IF, AG, MA, MM, FM, SV, DC, CM, and FP declare no competing interests.
Figures
References
-
- De Matteis E, Guglielmetti M, Ornello R, Spuntarelli V, Martelletti P, Sacco S (2020) Targeting CGRP for migraine treatment: mechanisms, antibodies, small molecules, perspectives. Expert Rev Neurother:1–15. 10.1080/14737175.2020.1772758 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous