Pharmacokinetic profile of amodiaquine and its active metabolite desethylamodiaquine in Ghanaian patients with uncomplicated falciparum malaria
- PMID: 33407454
- PMCID: PMC7788723
- DOI: 10.1186/s12936-020-03553-6
Pharmacokinetic profile of amodiaquine and its active metabolite desethylamodiaquine in Ghanaian patients with uncomplicated falciparum malaria
Erratum in
-
Correction to: Pharmacokinetic profile of amodiaquine and its active metabolite desethylamodiaquine in Ghanaian patients with uncomplicated falciparum malaria.Malar J. 2021 Mar 19;20(1):156. doi: 10.1186/s12936-021-03651-z. Malar J. 2021. PMID: 33740967 Free PMC article. No abstract available.
Abstract
Background: Accurate measurement of anti-malarial drug concentrations in therapeutic efficacy studies is essential to distinguish between inadequate drug exposure and anti-malarial drug resistance, and to inform optimal anti-malarial dosing in key target population groups.
Methods: A sensitive and selective LC-MS/MS method was developed and validated for the simultaneous determination of amodiaquine and its active metabolite, desethylamodiaquine, and used to describe their pharmacokinetic parameters in Ghanaian patients with uncomplicated falciparum malaria treated with the fixed-dose combination, artesunate-amodiaquine.
Results: The day-28 genotype-adjusted adequate clinical and parasitological response rate in 308 patients studied was > 97% by both intention-to-treat and per-protocol analysis. After excluding 64 patients with quantifiable amodiaquine concentrations pre-treatment and 17 with too few quantifiable concentrations, the pharmacokinetic analysis included 227 patients (9 infants, 127 aged 1-4 years, 91 aged ≥ 5 years). Increased median day-3 amodiaquine concentrations were associated with a lower risk of treatment failure [HR 0.87 (95% CI 0.78-0.98), p = 0.021]. Amodiaquine exposure (median AUC0-∞) was significantly higher in infants (4201 ng h/mL) and children aged 1-5 years (1994 ng h/mL) compared to older children and adults (875 ng h/mL, p = 0.001), even though infants received a lower mg/kg amodiaquine dose (median 25.3 versus 33.8 mg/kg in older patients). Desethylamodiaquine AUC0-∞ was not significantly associated with age. No significant safety concerns were identified.
Conclusions: Efficacy of artesunate-amodiaquine at currently recommended dosage regimens was high across all age groups. Reassuringly, amodiaquine and desethylamodiaquine exposure was not reduced in underweight-for-age young children or those with high parasitaemia, two of the most vulnerable target populations. A larger pharmacokinetic study with close monitoring of safety, including full blood counts and liver function tests, is needed to confirm the higher amodiaquine exposure in infants, understand any safety implications and assess whether dose optimization in this vulnerable, understudied population is needed.
Keywords: Amodiaquine; Artesunate; Fixed-dose combination; Ghana; Infants; P. falciparum malaria; Parasite density; Pharmacokinetics; Underweight-for-age; Young children.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
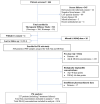
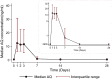
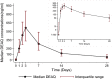


References
-
- WHO. World malaria report 2019. Geneva: World Health Organization; 2019. https://www.who.int/publications/i/item/9789241565721. Accessed 5 Nov 2020.
-
- WHO. Guidelines for the treatment of malaria. 3rd Edn. Geneva: World Health Organization; 2015. https://www.who.int/docs/default-source/documents/publications/gmp/guide.... Accessed 5 Nov 2020.
-
- Ghana Ministry of Health. Anti-Malaria Drug Policy for Ghana. Ministry of Health; 2009. https://www.ghanahealthservice.org/downloads/GHS_Antimalaria_drug_policy.... Accessed 5 Nov 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

