Resistin-like molecule β acts as a mitogenic factor in hypoxic pulmonary hypertension via the Ca2+-dependent PI3K/Akt/mTOR and PKC/MAPK signaling pathways
- PMID: 33407472
- PMCID: PMC7789700
- DOI: 10.1186/s12931-020-01598-4
Resistin-like molecule β acts as a mitogenic factor in hypoxic pulmonary hypertension via the Ca2+-dependent PI3K/Akt/mTOR and PKC/MAPK signaling pathways
Abstract
Background: Pulmonary arterial smooth muscle cell (PASMC) proliferation plays a crucial role in hypoxia-induced pulmonary hypertension (HPH). Previous studies have found that resistin-like molecule β (RELM-β) is upregulated de novo in response to hypoxia in cultured human PASMCs (hPASMCs). RELM-β has been reported to promote hPASMC proliferation and is involved in pulmonary vascular remodeling in patients with PAH. However, the expression pattern, effects, and mechanisms of action of RELM-β in HPH remain unclear.
Methods: We assessed the expression pattern, mitogenetic effect, and mechanism of action of RELM-β in a rat HPH model and in hPASMCs.
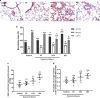
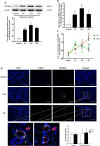
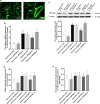
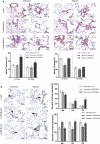
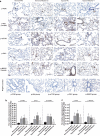
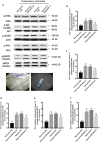
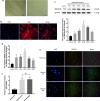
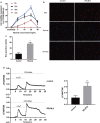
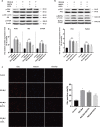
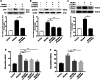
Results: Overexpression of RELM-β caused hemodynamic changes in a rat model of HPH similar to those induced by chronic hypoxia, including increased mean right ventricular systolic pressure (mRVSP), right ventricular hypertrophy index (RVHI) and thickening of small pulmonary arterioles. Knockdown of RELM-β partially blocked the increases in mRVSP, RVHI, and vascular remodeling induced by hypoxia. The phosphorylation levels of the PI3K, Akt, mTOR, PKC, and MAPK proteins were significantly up- or downregulated by RELM-β gene overexpression or silencing, respectively. Recombinant RELM-β protein increased the intracellular Ca2+ concentration in primary cultured hPASMCs and promoted hPASMC proliferation. The mitogenic effects of RELM-β on hPASMCs and the phosphorylation of PI3K, Akt, mTOR, PKC, and MAPK were suppressed by a Ca2+ inhibitor.
Conclusions: Our findings suggest that RELM-β acts as a cytokine-like growth factor in the development of HPH and that the effects of RELM-β are likely to be mediated by the Ca2+-dependent PI3K/Akt/mTOR and PKC/MAPK pathways.
Keywords: Ca2+; Hypoxic pulmonary arterial hypertension; Pulmonary vascular remodeling; Resistin-like molecule β; SOCE; Signaling pathway.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Opitz I, Ulrich S. Pulmonary hypertension in chronic obstructive pulmonary disease and emphysema patients: prevalence, therapeutic options and pulmonary circulatory effects of lung volume reduction surgery. J Thorac Dis. 2018;10(Suppl 23):S2763–S2774. doi: 10.21037/jtd.2018.07.63. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

