Multi-omic signatures of atherogenic dyslipidaemia: pre-clinical target identification and validation in humans
- PMID: 33407555
- PMCID: PMC7789501
- DOI: 10.1186/s12967-020-02663-8
Multi-omic signatures of atherogenic dyslipidaemia: pre-clinical target identification and validation in humans
Abstract
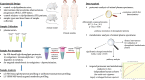
Background: Dyslipidaemia is a major risk factor for atherosclerosis and cardiovascular diseases. The molecular mechanisms that translate dyslipidaemia into atherogenesis and reliable markers of its progression are yet to be fully elucidated. To address this issue, we conducted a comprehensive metabolomic and proteomic analysis in an experimental model of dyslipidaemia and in patients with familial hypercholesterolemia (FH).
Methods: Liquid chromatography/mass spectrometry (LC/MS) and immunoassays were used to find out blood alterations at metabolite and protein levels in dyslipidaemic ApoE-/-/LDLR-/- mice and in FH patients to evaluate their human relevance.
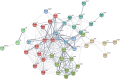
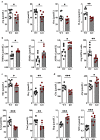
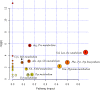
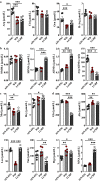
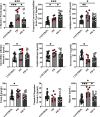
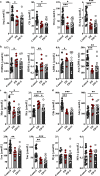
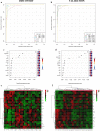
Results: We identified 15 metabolites (inhibitors and substrates of nitric oxide synthase (NOS), low-molecular-weight antioxidants (glutamine, taurine), homocysteine, methionine, 1-methylnicotinamide, alanine and hydroxyproline) and 9 proteins (C-reactive protein, proprotein convertase subtilisin/kexin type 9, apolipoprotein C-III, soluble intercellular adhesion molecule-1, angiotensinogen, paraoxonase-1, fetuin-B, vitamin K-dependent protein S and biglycan) that differentiated FH patients from healthy controls. Most of these changes were consistently found in dyslipidaemic mice and were further amplified if mice were fed an atherogenic (Western or low-carbohydrate, high-protein) diet.
Conclusions: The alterations highlighted the involvement of an immune-inflammatory response system, oxidative stress, hyper-coagulation and impairment in the vascular function/regenerative capacity in response to dyslipidaemia that may also be directly engaged in development of atherosclerosis. Our study further identified potential biomarkers for an increased risk of atherosclerosis that may aid in clinical diagnosis or in the personalized treatment.
Keywords: Atherosclerosis; Dyslipidaemia; Metabolome; Pathological mechanisms; Proteome; Serological biomarkers.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

