Early combination therapy with etanercept and methotrexate in JIA patients shortens the time to reach an inactive disease state and remission: results of a double-blind placebo-controlled trial
- PMID: 33407590
- PMCID: PMC7788754
- DOI: 10.1186/s12969-020-00488-9
Early combination therapy with etanercept and methotrexate in JIA patients shortens the time to reach an inactive disease state and remission: results of a double-blind placebo-controlled trial
Abstract
Background: Remission is the primary objective of treating juvenile idiopathic arthritis (JIA). It is still debatable whether early intensive treatment is superior in terms of earlier achievement of remission. The aim of this study was to evaluate the effectiveness of early etanercept+methotrexate (ETA+MTX) combination therapy versus step-up MTX monotherapy with ETA added in refractory disease.
Methods: A multi-centre, double-blind, randomized study in active polyarticular JIA patients treated with either ETA+MTX (n = 35) or placebo+MTX (n = 33) for up to 24 weeks, followed by a 24-week open-label phase. The efficacy endpoints included pedACR30 criteria improvement at week 12, inactive disease at week 24, and remission at week 48. Patients who failed to achieve the endpoints at week 12 or at week 24 escaped to open-label ETA+MTX. Safety was assessed at each visit.
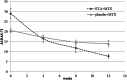
Results: By intention-to-treat analysis, more patients in the ETA+MTX group reached the pedACR30 response at week 12 (33 (94.3%)) than in the placebo+MTX group (20 (60.6%); p = 0.001). At week 24, comparable percentages of patients reached inactive disease (11 (31.4%) vs 11 (33.3%)). At week 48, 11 (31.4%) and eight (24.2%) patients achieved remission. The median (+/-IQR) times to achieve an inactive disease state in the ETA+MTX and placebo+MTX groups were 24 (14-32) and 32 (24-40) weeks, respectively. Forty-four (74/100 patient-years) adverse events (AEs) were reported, leading to treatment discontinuation in 6 patients.
Conclusions: Early combination therapy with ETA+MTX proved to be highly effective compared to the standard step-up regimen. Compared to those treated with the standard regimen, more patients treated with a combination of ETA+MTX reached the pedACR30 response and achieved inactive disease and remission more rapidly.
Keywords: Juvenile idiopathic arthritis; Remission; TNF inhibitor.
Conflict of interest statement
E. Alexeeva has received research funds, advisory board membership and honorary fees from Novartis, Pfizer, Sanofi, MSD, AMGEN, Eli Lilly, and Roche.
G. Horneff has received research funds, advisory board membership and honorary fees from Abbvie, Pfizer and Roche.
T. Dvoryakovskaya has received research funds, advisory board membership and honorary fees from Novartis, Pfizer, MSD, AMGEN, Eli Lilly, and Roche.
R. Denisova has received research funds, advisory board membership and honorary fees from Novartis, Pfizer, Sanofi, MSD, and Roche.
I. Nikishina has received research funds and honorary fees from Abbvie, Pfizer, Novartis, Roche, MSD.
E. Zholobova has received research funds from Pfizer and Novartis, speaker honoraria, including speaker bureau and symposia, and expert witness fees from Abbvie, Pfizer, Roche, and Novartis.
V. Malievskiy has received research grants from Pfizer, Janseen Research & Development LLC, speaker honoraria from Abbvie, Pfizer, Roche, and Novartis.
E. Stadler has received research funds and honorary grants from Abbvie, Pfizer and Roche.
L. Balykova has received research funds from Pfizer.
Y. Spivakovskiy has received research grants from Pfizer and Jansen Research and received honoraria as a speaker for Novartis and Pfizer.
G. Santalova, I. Kriulin, A. Alshevskaya, and A. Moskalev declare that they have no competing interests.
Figures


References
-
- Del Giudice E, Swart JF, Wulffraat NM. Juvenile idiopathic arthritis. Comorbidity Rheum Dis. 2017;5:265–288.
-
- Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–778. - PubMed
-
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, He X, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392. - PubMed
-
- Ringold S, Angeles-Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for non-systemic polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care Res (Hoboken) 2019;71(6):717–734. - PMC - PubMed
-
- Giannini EH, Brewer EJ, Kuzmina N, Shaikov A, Maximov A, Vorontsov I, et al. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.a.-U.S.S.R. double-blind, placebo-controlled trial. The pediatric rheumatology collaborative study group and the cooperative Children's study group. N Engl J Med. 1992;326(16):1043–1049. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

