Hypoxia and the phenomenon of immune exclusion
- PMID: 33407613
- PMCID: PMC7788724
- DOI: 10.1186/s12967-020-02667-4
Hypoxia and the phenomenon of immune exclusion
Abstract
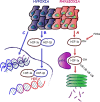
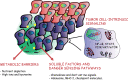
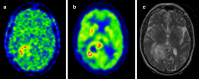
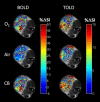
Over the last few years, cancer immunotherapy experienced tremendous developments and it is nowadays considered a promising strategy against many types of cancer. However, the exclusion of lymphocytes from the tumor nest is a common phenomenon that limits the efficiency of immunotherapy in solid tumors. Despite several mechanisms proposed during the years to explain the immune excluded phenotype, at present, there is no integrated understanding about the role played by different models of immune exclusion in human cancers. Hypoxia is a hallmark of most solid tumors and, being a multifaceted and complex condition, shapes in a unique way the tumor microenvironment, affecting gene transcription and chromatin remodeling. In this review, we speculate about an upstream role for hypoxia as a common biological determinant of immune exclusion in solid tumors. We also discuss the current state of ex vivo and in vivo imaging of hypoxic determinants in relation to T cell distribution that could mechanisms of immune exclusion and discover functional-morphological tumor features that could support clinical monitoring.
Keywords: Dynamic barriers; Functional barriers; Hypoxia; Imaging; Immune exclusion; Physical barriers; Tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Next Generation Imaging Techniques to Define Immune Topographies in Solid Tumors.Front Immunol. 2021 Jan 27;11:604967. doi: 10.3389/fimmu.2020.604967. eCollection 2020. Front Immunol. 2021. PMID: 33584676 Free PMC article. Review.
-
Metformin improves cancer immunotherapy by directly rescuing tumor-infiltrating CD8 T lymphocytes from hypoxia-induced immunosuppression.J Immunother Cancer. 2023 May;11(5):e005719. doi: 10.1136/jitc-2022-005719. J Immunother Cancer. 2023. PMID: 37147018 Free PMC article.
-
Targeting hypoxia in the tumor microenvironment: a potential strategy to improve cancer immunotherapy.J Exp Clin Cancer Res. 2021 Jan 9;40(1):24. doi: 10.1186/s13046-020-01820-7. J Exp Clin Cancer Res. 2021. PMID: 33422072 Free PMC article. Review.
-
Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer.Oncogene. 2017 Jan 26;36(4):439-445. doi: 10.1038/onc.2016.225. Epub 2016 Jun 27. Oncogene. 2017. PMID: 27345407 Free PMC article. Review.
-
Hypoxia as a potential inducer of immune tolerance, tumor plasticity and a driver of tumor mutational burden: Impact on cancer immunotherapy.Semin Cancer Biol. 2023 Dec;97:104-123. doi: 10.1016/j.semcancer.2023.11.008. Epub 2023 Nov 28. Semin Cancer Biol. 2023. PMID: 38029865 Review.
Cited by
-
Tumour Hypoxia-Mediated Immunosuppression: Mechanisms and Therapeutic Approaches to Improve Cancer Immunotherapy.Cells. 2021 Apr 24;10(5):1006. doi: 10.3390/cells10051006. Cells. 2021. PMID: 33923305 Free PMC article. Review.
-
The potential longevity-promoting hypoxic-hypercapnic environment as a measure for radioprotection.Biogerontology. 2024 Oct;25(5):891-898. doi: 10.1007/s10522-024-10129-3. Epub 2024 Aug 20. Biogerontology. 2024. PMID: 39162980 Free PMC article. Review.
-
Mechanisms of HIF-driven immunosuppression in tumour microenvironment.J Egypt Natl Canc Inst. 2023 Aug 30;35(1):27. doi: 10.1186/s43046-023-00186-z. J Egypt Natl Canc Inst. 2023. PMID: 37646847 Review.
-
Development and validation of a hypoxia-associated signature for lung adenocarcinoma.Sci Rep. 2022 Jan 25;12(1):1290. doi: 10.1038/s41598-022-05385-7. Sci Rep. 2022. PMID: 35079065 Free PMC article.
-
Effects of combination of obesity, diabetes, and hypoxia on inflammatory regulating genes and cytokines in rat pancreatic tissues and serum.PeerJ. 2022 Oct 4;10:e13990. doi: 10.7717/peerj.13990. eCollection 2022. PeerJ. 2022. PMID: 36213511 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

