Amniotic fluid-derived multipotent stromal cells drive diabetic wound healing through modulation of macrophages
- PMID: 33407615
- PMCID: PMC7789548
- DOI: 10.1186/s12967-020-02674-5
Amniotic fluid-derived multipotent stromal cells drive diabetic wound healing through modulation of macrophages
Abstract
Background: Cutaneous wounds in patients with diabetes exhibit impaired healing due to physiological impediments and conventional care options are severely limited. Multipotent stromal cells (MSCs) have been touted as a powerful new therapy for diabetic tissue repair owing to their trophic activity and low immunogenicity. However, variations in sources and access are limiting factors for broader adaptation and study of MSC-based therapies. Amniotic fluid presents a relatively unexplored source of MSCs and one with wide availability. Here, we investigate the potential of amniotic fluid-derived multipotent stromal cells (AFMSCs) to restore molecular integrity to diabetic wounds, amend pathology and promote wound healing.
Method: We obtained third trimester amniotic fluid from term cesarean delivery and isolated and expanded MSCs in vitro. We then generated 10 mm wounds in Leprdb/db diabetic mouse skin, and splinted them open to allow for humanized wound modeling. Immediately after wounding, we applied AFMSCs topically to the sites of injuries on diabetic mice, while media application only, defined as vehicle, served as controls. Post-treatment, we compared healing time and molecular and cellular events of AFMSC-treated, vehicle-treated, untreated diabetic, and non-diabetic wounds. A priori statistical analyses measures determined significance of the data.
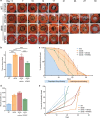
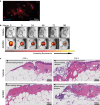
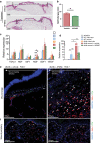
Result: Average time to wound closure was approximately 19 days in AFMSC-treated diabetic wounds. This was significantly lower than the vehicle-treated diabetic wounds, which required on average 27.5 days to heal (p < 0.01), and most similar to time of closure in wild type untreated wounds (an average of around 18 days). In addition, AFMSC treatment induced changes in the profiles of macrophage polarizing cytokines, resulting in a change in macrophage composition in the diabetic wound bed. We found no evidence of AFMSC engraftment or biotherapy induced immune response.
Conclusion: Treatment of diabetic wounds using amniotic fluid-derived MSCs encourages cutaneous tissue repair through affecting inflammatory cell behavior in the wound site. Since vehicle-treated diabetic wounds did not demonstrate accelerated healing, we determined that AFMSCs were therapeutic through their paracrine activities. Future studies should be aimed towards validating our observations through further examination of the paracrine potential of AFMSCs. In addition, investigations concerning safety and efficacy of this therapy in clinical trials should be pursued.
Keywords: Amniotic fluid multipotent stromal cells; Cellular therapy; Diabetic wounds.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice.PLoS One. 2017 Jun 8;12(6):e0177533. doi: 10.1371/journal.pone.0177533. eCollection 2017. PLoS One. 2017. PMID: 28594903 Free PMC article.
-
Mesenchymal stem cells-derived MFG-E8 accelerates diabetic cutaneous wound healing.J Dermatol Sci. 2017 Jun;86(3):187-197. doi: 10.1016/j.jdermsci.2017.02.285. Epub 2017 Mar 6. J Dermatol Sci. 2017. PMID: 28302404
-
Skin-derived precursor cells promote wound healing in diabetic mice.Ann Plast Surg. 2015 Jan;74(1):114-20. doi: 10.1097/SAP.0000000000000342. Ann Plast Surg. 2015. PMID: 25188249
-
Cutaneous Wound Healing and the Effects of Cannabidiol.Int J Mol Sci. 2024 Jun 28;25(13):7137. doi: 10.3390/ijms25137137. Int J Mol Sci. 2024. PMID: 39000244 Free PMC article. Review.
-
The role of adipose tissue-derived stromal cells, macrophages and bioscaffolds in cutaneous wound repair.Biol Direct. 2024 Sep 29;19(1):85. doi: 10.1186/s13062-024-00534-6. Biol Direct. 2024. PMID: 39343924 Free PMC article. Review.
Cited by
-
Perinatal Stem Cell Therapy to Treat Type 1 Diabetes Mellitus: A Never-Say-Die Story of Differentiation and Immunomodulation.Int J Mol Sci. 2022 Nov 23;23(23):14597. doi: 10.3390/ijms232314597. Int J Mol Sci. 2022. PMID: 36498923 Free PMC article. Review.
-
Tibial cortex transverse transport promotes ischemic diabetic foot ulcer healing via enhanced angiogenesis and inflammation modulation in a novel rat model.Eur J Med Res. 2024 Mar 6;29(1):155. doi: 10.1186/s40001-024-01752-4. Eur J Med Res. 2024. PMID: 38449025 Free PMC article.
-
Behind the Scenes of Extracellular Vesicle Therapy for Skin Injuries and Disorders.Adv Wound Care (New Rochelle). 2022 Nov;11(11):575-597. doi: 10.1089/wound.2021.0066. Epub 2021 Dec 30. Adv Wound Care (New Rochelle). 2022. PMID: 34806432 Free PMC article. Review.
-
Panthenol Citrate Biomaterials Accelerate Wound Healing and Restore Tissue Integrity.Adv Healthc Mater. 2023 Dec;12(31):e2301683. doi: 10.1002/adhm.202301683. Epub 2023 Jun 25. Adv Healthc Mater. 2023. PMID: 37327023 Free PMC article.
-
Exosome Loaded Protein Hydrogel for Enhanced Gelation Kinetics and Wound Healing.ACS Appl Bio Mater. 2024 Sep 16;7(9):5992-6000. doi: 10.1021/acsabm.4c00569. Epub 2024 Aug 22. ACS Appl Bio Mater. 2024. PMID: 39173187 Free PMC article.
References
-
- National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention, US Department of Health and Human Services, Atlanta, GA. https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Accessed 19 May 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

