Anti-CD40 antibody KPL-404 inhibits T cell-mediated activation of B cells from healthy donors and autoimmune patients
- PMID: 33407802
- PMCID: PMC7789801
- DOI: 10.1186/s13075-020-02372-z
Anti-CD40 antibody KPL-404 inhibits T cell-mediated activation of B cells from healthy donors and autoimmune patients
Erratum in
-
Correction to: Anti-CD40 antibody KPL-404 inhibits T cell-mediated activation of B cells from healthy donors and autoimmune patients.Arthritis Res Ther. 2021 Jan 21;23(1):36. doi: 10.1186/s13075-021-02425-x. Arthritis Res Ther. 2021. PMID: 33478546 Free PMC article. No abstract available.
Abstract
Background: CD40-CD40L is a key co-stimulatory pathway for B cell activation. As such, its blockade can inhibit pathogenic B cell responses in autoimmune diseases, such as Sjogren's syndrome (SjS) and systemic lupus erythematosus (SLE). In this study, we examined the in vitro effects of KPL-404, a humanized anti-CD40 monoclonal antibody (Ab), on primary human B cells derived from either healthy donors (HD) or autoimmune patients and compared them to the effects of G28-5, a partially antagonistic anti-CD40 antibody.
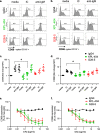
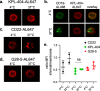
Methods: PBMCs from HD or SjS and SLE patients were cultured in high-density cell cultures in the presence of IgG4 isotype control or anti-CD40 Abs KPL-404 or G28-5. Cells were stimulated with anti-CD3/CD28 cross-linking reagent ImmunoCult (IC) to induce CD40L-CD40-mediated B cell responses. B cell proliferation and activation, measured by dilution of proliferation tracker dye and the upregulation of CD69 and CD86, respectively, were assessed by flow cytometry. Anti-CD40 Ab cell-internalization was examined by imaging flow cytometry. Cytokine release in the PBMC cultures was quantified by bead-based multiplex assay.
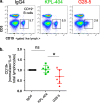
Results: KPL-404 binds to CD40 expressed on different subsets of B cells without inducing cell depletion, or B cell proliferation and activation in in vitro culture. Under the same conditions, G28-5 promoted proliferation of and increased CD69 expression on otherwise unstimulated B cells. KPL-404 efficiently blocked the CD40L-CD40-mediated activation of B cells from HD at concentrations between 1 and 10 μg/ml. Treatment with KPL-404 alone did not promote cytokine production and blocked the production of IFNβ in healthy PBMC cultures. KPL-404 efficiently blocked CD40L-CD40-mediated activation of B cells from patients with SjS and SLE, without affecting their anti-IgM responses or affecting their cytokine production. Consistent with the differences of their effects on B cell responses, KPL-404 was not internalized by cells, whereas G28-5 showed partial internalization upon CD40 binding.
Conclusions: Anti-CD40 mAb KPL-404 showed purely antagonistic effects on B cells and total PBMCs. KPL-404 inhibited CD40L-CD40-mediated B cell activation in PBMC cultures from both healthy controls and autoimmune patients. These data support the therapeutic potential of CD40 targeting by KPL-404 Ab for inhibiting B cell responses in SjS and SLE.
Keywords: Anti-CD40 mAb; B cell activation; CD40-CD40L co-stimulation; KPL-404; Sjogren’s syndrome (SjS); Systemic lupus erythematosus (SLE).
Conflict of interest statement
This study was supported by Kiniksa Pharmaceuticals, Ltd. S.M. is an employee of Kiniksa Pharmaceuticals Corp. and J.M and N.G. received research funding from Kiniksa Pharmaceuticals, Ltd.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

