Micro-RNA-125a mediates the effects of hypomethylating agents in chronic myelomonocytic leukemia
- PMID: 33407852
- PMCID: PMC7789782
- DOI: 10.1186/s13148-020-00979-2
Micro-RNA-125a mediates the effects of hypomethylating agents in chronic myelomonocytic leukemia
Abstract
Background: Chronic myelomonocytic leukemia (CMML) is an aggressive hematopoietic malignancy that arises from hematopoietic stem and progenitor cells (HSPCs). Patients with CMML are frequently treated with epigenetic therapeutic approaches, in particular the hypomethylating agents (HMAs), azacitidine (Aza) and decitabine (Dec). Although HMAs are believed to mediate their efficacy via re-expression of hypermethylated tumor suppressors, knowledge about relevant HMA targets is scarce. As silencing of tumor-suppressive micro-RNAs (miRs) by promoter hypermethylation is a crucial step in malignant transformation, we asked for a role of miRs in HMA efficacy in CMML.
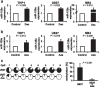
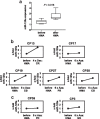
Results: Initially, we performed genome-wide miR-expression profiling in a KrasG12D-induced CMML mouse model. Selected candidates with prominently decreased expression were validated by qPCR in CMML mice and human CMML patients. These experiments revealed the consistent decrease in miR-125a, a miR with previously described tumor-suppressive function in myeloid neoplasias. Furthermore, we show that miR-125a downregulation is caused by hypermethylation of its upstream region and can be reversed by HMA treatment. By employing both lentiviral and CRISPR/Cas9-based miR-125a modification, we demonstrate that HMA-induced miR-125a upregulation indeed contributes to mediating the anti-leukemic effects of these drugs. These data were validated in a clinical context, as miR-125a expression increased after HMA treatment in CMML patients, a phenomenon that was particularly pronounced in cases showing clinical response to these drugs.
Conclusions: Taken together, we report decreased expression of miR-125a in CMML and delineate its relevance as mediator of HMA efficacy within this neoplasia.
Keywords: Azacitidine; Chronic myelomonocytic leukemia; Hypomethylating agent; Tumor suppressor; miRNA.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


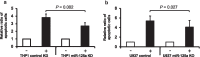
References
-
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. - PubMed
-
- Itzykson R, Fenaux P, Bowen D, Cross NCP, Cortes J, De Witte T, et al. Diagnosis and treatment of chronic myelomonocytic leukemias in adults: recommendations from the European Hematology Association and the European LeukemiaNet. Hemasphere. 2018;2:e150. doi: 10.1097/HS9.0000000000000150. - DOI - PMC - PubMed
-
- Geissler K, Jager E, Barna A, Gurbisz M, Marschon R, Graf T, et al. The Austrian biodatabase for chronic myelomonocytic leukemia (ABCMML): a representative and useful real-life data source for further biomedical research. Wien Klin Wochenschr. 2019;131:410–418. doi: 10.1007/s00508-019-1526-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

