Hypoxic hUCMSC-derived extracellular vesicles attenuate allergic airway inflammation and airway remodeling in chronic asthma mice
- PMID: 33407872
- PMCID: PMC7789736
- DOI: 10.1186/s13287-020-02072-0
Hypoxic hUCMSC-derived extracellular vesicles attenuate allergic airway inflammation and airway remodeling in chronic asthma mice
Abstract
Background: As one of the main functional forms of mesenchymal stem cells (MSCs), MSC-derived extracellular vesicles (MSC-EVs) have shown an alternative therapeutic option in experimental models of allergic asthma. Oxygen concentration plays an important role in the self-renewal, proliferation, and EV release of MSCs and a recent study found that the anti-asthma effect of MSCs was enhanced by culture in hypoxic conditions. However, the potential of hypoxic MSC-derived EVs (Hypo-EVs) in asthma is still unknown.
Methods: BALB/c female mice were sensitized and challenged with ovalbumin (OVA), and each group received PBS, normoxic human umbilical cord MSC-EVs (Nor-EVs), or Hypo-EVs weekly. After treatment, the animals were euthanized, and their lungs and bronchoalveolar lavage fluid (BALF) were collected. With the use of hematoxylin and eosin (HE), periodic acid-Schiff (PAS) and Masson's trichrome staining, enzyme-linked immune sorbent assay (ELISA), Western blot analysis, and real-time PCR, the inflammation and collagen fiber content of airways and lung parenchyma were investigated.
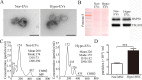
Results: Hypoxic environment can promote human umbilical cord MSCs (hUCMSCs) to release more EVs. In OVA animals, the administration of Nor-EVs or Hypo-EVs significantly ameliorated the BALF total cells, eosinophils, and pro-inflammatory mediators (IL-4 and IL-13) in asthmatic mice. Moreover, Hypo-EVs were generally more potent than Nor-EVs in suppressing airway inflammation in asthmatic mice. Compared with Nor-EVs, Hypo-EVs further prevented mouse chronic allergic airway remodeling, concomitant with the decreased expression of pro-fibrogenic markers α-smooth muscle actin (α-SMA), collagen-1, and TGF-β1-p-smad2/3 signaling pathway. In vitro, Hypo-EVs decreased the expression of p-smad2/3, α-SMA, and collagen-1 in HLF-1 cells (human lung fibroblasts) stimulated by TGF-β1. In addition, we showed that miR-146a-5p was enriched in Hypo-EVs compared with that in Nor-EVs, and Hypo-EV administration unregulated the miR-146a-5p expression both in asthma mice lung tissues and in TGF-β1-treated HLF-1. More importantly, decreased miR-146a-5p expression in Hypo-EVs impaired Hypo-EV-mediated lung protection in OVA mice.
Conclusion: Our findings provided the first evidence that hypoxic hUCMSC-derived EVs attenuated allergic airway inflammation and airway remodeling in chronic asthma mice, potentially creating new avenues for the treatment of asthma.
Keywords: Asthma; Extracellular vesicles; Human umbilical cord mesenchymal stem cells; Hypoxia; Lung injury.
Conflict of interest statement
There are no conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

