Constructing an ethical framework for priority allocation of pandemic vaccines
- PMID: 33408013
- PMCID: PMC7779078
- DOI: 10.1016/j.vaccine.2020.12.053
Constructing an ethical framework for priority allocation of pandemic vaccines
Abstract
Background: Allocation of scarce resources during a pandemic extends to the allocation of vaccines when they eventually become available. We describe a framework for priority vaccine allocation that employed a cross-disciplinary approach, guided by ethical considerations and informed by local risk assessment.
Methods: Published and grey literature was reviewed, and augmented by consultation with key informants, to collate past experience, existing guidelines and emerging strategies for pandemic vaccine deployment. Identified ethical issues and decision-making processes were also included. Concurrently, simulation modelling studies estimated the likely impacts of alternative vaccine allocation approaches. Assembled evidence was presented to a workshop of national experts in pandemic preparedness, vaccine strategy, implementation and ethics. All of this evidence was then used to generate a proposed ethical framework for vaccine priorities best suited to the Australian context.
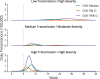
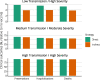
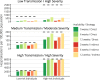
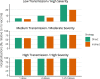
Findings: Published and emerging guidance for priority pandemic vaccine distribution differed widely with respect to strategic objectives, specification of target groups, and explicit discussion of ethical considerations and decision-making processes. Flexibility in response was universally emphasised, informed by real-time assessment of the pandemic impact level, and identification of disproportionately affected groups. Model outputs aided identification of vaccine approaches most likely to achieve overarching goals in pandemics of varying transmissibility and severity. Pandemic response aims deemed most relevant for an Australian framework were: creating and maintaining trust, promoting equity, and reducing harmful outcomes.
Interpretation: Defining clear and ethically-defendable objectives for pandemic response in context aids development of flexible and adaptive decision support frameworks and facilitates clear communication and engagement activities.
Keywords: Pandemic vaccine; Priority populations; Vaccine allocation; Vaccine ethics.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Risk management frameworks for human health and environmental risks.J Toxicol Environ Health B Crit Rev. 2003 Nov-Dec;6(6):569-720. doi: 10.1080/10937400390208608. J Toxicol Environ Health B Crit Rev. 2003. PMID: 14698953 Review.
-
Ethical values and principles to guide the fair allocation of resources in response to a pandemic: a rapid systematic review.BMC Med Ethics. 2022 Jul 7;23(1):70. doi: 10.1186/s12910-022-00806-8. BMC Med Ethics. 2022. PMID: 35799187 Free PMC article.
-
Ethics-sensitivity of the Ghana national integrated strategic response plan for pandemic influenza.BMC Med Ethics. 2015 May 7;16:30. doi: 10.1186/s12910-015-0025-9. BMC Med Ethics. 2015. PMID: 25947354 Free PMC article.
-
Pandemic influenza preparedness: an ethical framework to guide decision-making.BMC Med Ethics. 2006 Dec 4;7:E12. doi: 10.1186/1472-6939-7-12. BMC Med Ethics. 2006. PMID: 17144926 Free PMC article.
-
Achieving Global Vaccine Equity: The Case for an International Pandemic Treaty.Yale J Biol Med. 2022 Jun 30;95(2):271-280. eCollection 2022 Jun. Yale J Biol Med. 2022. PMID: 35782474 Free PMC article. Review.
Cited by
-
Priority allocation of pandemic influenza vaccines in Australia - Recommendations of 3 community juries.Vaccine. 2021 Jan 8;39(2):255-262. doi: 10.1016/j.vaccine.2020.12.010. Epub 2020 Dec 13. Vaccine. 2021. PMID: 33317870 Free PMC article.
-
Racial disparities in Phase 1 COVID-19 vaccine shipments to Neighborhood sites in Pennsylvania by the Federal Retail Pharmacy Program.Sci Rep. 2024 Oct 10;14(1):23591. doi: 10.1038/s41598-024-73116-1. Sci Rep. 2024. PMID: 39390039 Free PMC article.
-
Having a real say: findings from first nations community panels on pandemic influenza vaccine distribution.BMC Public Health. 2023 Nov 30;23(1):2377. doi: 10.1186/s12889-023-17262-7. BMC Public Health. 2023. PMID: 38037021 Free PMC article.
-
COVID-19 vaccination preferences during a pause in Johnson & Johnson vaccine administration.Vaccine X. 2023 Aug 18;15:100373. doi: 10.1016/j.jvacx.2023.100373. eCollection 2023 Dec. Vaccine X. 2023. PMID: 37674932 Free PMC article.
References
-
- Akst J. RNA Extraction Kits for COVID-19 Tests Are in Short Supply in US. The Scientist. 2020 March 11.
-
- Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A., et al. Fair allocation of scarce medical resources in the time of covid-19. N Engl J Med. 2020 - PubMed
-
- Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages — the need for ventilators and personal protective equipment during the covid-19 pandemic. N Engl J Med. 2020 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

