A muscle growth-promoting treatment based on the attenuation of activin/myostatin signalling results in long-term testicular abnormalities
- PMID: 33408083
- PMCID: PMC7903914
- DOI: 10.1242/dmm.047555
A muscle growth-promoting treatment based on the attenuation of activin/myostatin signalling results in long-term testicular abnormalities
Abstract
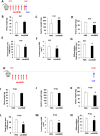
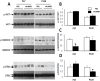
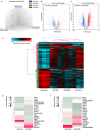
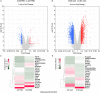
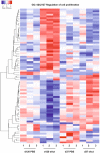
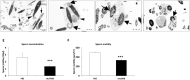
Activin/myostatin signalling acts to induce skeletal muscle atrophy in adult mammals by inhibiting protein synthesis as well as promoting protein and organelle turnover. Numerous strategies have been successfully developed to attenuate the signalling properties of these molecules, which result in augmenting muscle growth. However, these molecules, in particular activin, play major roles in tissue homeostasis in numerous organs of the mammalian body. We have recently shown that although the attenuation of activin/myostatin results in robust muscle growth, it also has a detrimental impact on the testis. Here, we aimed to discover the long-term consequences of a brief period of exposure to muscle growth-promoting molecules in the testis. We demonstrate that muscle hypertrophy promoted by a soluble activin type IIB ligand trap (sActRIIB) is a short-lived phenomenon. In stark contrast, short-term treatment with sActRIIB results in immediate impact on the testis, which persists after the sessions of the intervention. Gene array analysis identified an expansion in aberrant gene expression over time in the testis, initiated by a brief exposure to muscle growth-promoting molecules. The impact on the testis results in decreased organ size as well as quantitative and qualitative impact on sperm. Finally, we have used a drug-repurposing strategy to exploit the gene expression data to identify a compound - N6-methyladenosine - that may protect the testis from the impact of the muscle growth-promoting regime. This work indicates the potential long-term harmful effects of strategies aimed at promoting muscle growth by attenuating activin/myostatin signalling. Furthermore, we have identified a molecule that could, in the future, be used to overcome the detrimental impact of sActRIIB treatment on the testis.
Keywords: Activin; Gene array; Muscle; Myostatin; Testis.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


Similar articles
-
Compression of morbidity in a progeroid mouse model through the attenuation of myostatin/activin signalling.J Cachexia Sarcopenia Muscle. 2019 Jun;10(3):662-686. doi: 10.1002/jcsm.12404. Epub 2019 Mar 27. J Cachexia Sarcopenia Muscle. 2019. PMID: 30916493 Free PMC article.
-
Myostatin/activin blocking combined with exercise reconditions skeletal muscle expression profile of mdx mice.Mol Cell Endocrinol. 2015 Jan 5;399:131-42. doi: 10.1016/j.mce.2014.10.001. Epub 2014 Oct 7. Mol Cell Endocrinol. 2015. PMID: 25304272
-
Inhibition of Activin/Myostatin signalling induces skeletal muscle hypertrophy but impairs mouse testicular development.Eur J Transl Myol. 2020 Apr 1;30(1):8737. doi: 10.4081/ejtm.2019.8737. eCollection 2020 Apr 7. Eur J Transl Myol. 2020. PMID: 32499882 Free PMC article.
-
Interference with myostatin/ActRIIB signaling as a therapeutic strategy for Duchenne muscular dystrophy.Curr Gene Ther. 2012 Jun;12(3):245-59. doi: 10.2174/156652312800840577. Curr Gene Ther. 2012. PMID: 22554312 Review.
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
Cited by
-
Study on the relationship between NF-kB pathway and skeletal muscle dopamine receptors in muscle-attenuated mice.Afr Health Sci. 2024 Sep;24(3):438-443. doi: 10.4314/ahs.v24i3.48. Afr Health Sci. 2024. PMID: 40777974 Free PMC article.
-
Targeting the Activin Receptor Signaling to Counteract the Multi-Systemic Complications of Cancer and Its Treatments.Cells. 2021 Feb 28;10(3):516. doi: 10.3390/cells10030516. Cells. 2021. PMID: 33671024 Free PMC article. Review.
References
-
- Alyodawi, K., Vermeij, W. P., Omairi, S., Kretz, O., Hopkinson, M., Solagna, F., Joch, B., Brandt, R. M. C., Barnhoorn, S., van Vliet, N.et al. (2019). Compression of morbidity in a progeroid mouse model through the attenuation of myostatin/activin signalling. J. Cachexia Sarcopenia Muscle 10, 662-686. 10.1002/jcsm.12404 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

