Effect of Ocrelizumab in Blood Leukocytes of Patients With Primary Progressive MS
- PMID: 33408167
- PMCID: PMC7862094
- DOI: 10.1212/NXI.0000000000000940
Effect of Ocrelizumab in Blood Leukocytes of Patients With Primary Progressive MS
Abstract
Objective: To analyze the changes induced by ocrelizumab in blood immune cells of patients with primary progressive MS (PPMS).
Methods: In this multicenter prospective study including 53 patients with PPMS who initiated ocrelizumab treatment, we determined effector, memory, and regulatory cells by flow cytometry at baseline and after 6 months of therapy. Wilcoxon matched paired tests were used to assess differences between baseline and 6 months' results. p Values were corrected using the Bonferroni test.
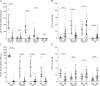
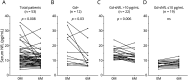
Results: Ocrelizumab reduced the numbers of naive and memory B cells (p < 0.0001) and those of B cells producing interleukin (IL)-6, IL-10, granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor-alpha (TNFα) (p < 0.0001 in all cases). By contrast, the proportions of plasmablasts and B cells producing GM-CSF and TNFα increased significantly, suggesting the need for treatment continuation. We also observed a decrease in CD20+ T-cell numbers (p < 0.0001) and percentages (p < 0.0001), and a clear remodeling of the T-cell compartment characterized by relative increases of the naive/effector ratios in CD4+ (p = 0.002) and CD8+ (p = 0.002) T cells and relative decreases of CD4+ (p = 0.03) and CD8+ (p = 0.004) T cells producing interferon-gamma. Total monocyte numbers increased (p = 0.002), but no changes were observed in those producing inflammatory cytokines. The immunologic variations were associated with a reduction of serum neurofilament light chain (sNfL) levels (p = 0.008). The reduction was observed in patients with Gd-enhanced lesions at baseline and in Gd- patients with baseline sNfL >10 pg/mL.
Conclusions: In PPMS, effector B-cell depletion changed T-cell response toward a low inflammatory profile, resulting in decreased sNfL levels.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

References
-
- Faissner S, Plemel J, Gold R, Yong W. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov 2019;18:905–922. - PubMed
-
- Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Eng J Med 2017;376:209–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
