Efficacy of Coxsackievirus A5 Vaccine Candidates in an Actively Immunized Mouse Model
- PMID: 33408178
- PMCID: PMC8094937
- DOI: 10.1128/JVI.01743-20
Efficacy of Coxsackievirus A5 Vaccine Candidates in an Actively Immunized Mouse Model
Abstract
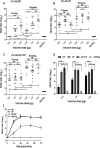
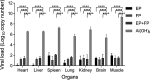
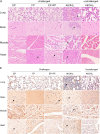
Coxsackievirus A5 (CV-A5) has recently emerged as a main hand, foot, and mouth disease (HFMD) pathogen. Following a large-scale vaccination campaign against enterovirus 71 (EV-71) in China, the number of HFMD-associated cases with EV-71 was reduced, especially severe and fatal cases. However, the total number of HFMD cases remains high, as HFMD is also caused by other enterovirus serotypes. A multivalent HFMD vaccine containing 4 or 6 antigens of enterovirus serotypes is urgently needed. A formaldehyde-inactivated CV-A5 vaccine derived from Vero cells was used to inoculate newborn Kunming mice on days 3 and 10. The mice were challenged on day 14 with a mouse-adapted CV-A5 strain at a dose that was lethal for 14-day-old suckling mice. Within 14 days postchallenge, groups of mice immunized with three formulations, empty particles (EPs), full particles (FPs), and a mixture of the EP and FP vaccine candidates, all survived, while 100% of the mock-immunized mice died. Neutralizing antibodies (NtAbs) were detected in the sera of immunized mice, and the NtAb levels were correlated with the survival rate of the challenged mice. The virus loads in organs were reduced, and pathological changes and viral protein expression were weak or not observed in the immunized mice compared with those in alum-inoculated control mice. Another interesting finding was the identification of CV-A5 dense particles (DPs), facilitating morphogenesis study. These results demonstrated that the Vero cell-adapted CV-A5 strain is a promising vaccine candidate and could be used as a multivalent HFMD vaccine component in the future.IMPORTANCE The vaccine candidate strain CV-A5 was produced with a high infectivity titer and a high viral particle yield. Three particle forms, empty particles (EPs), full particles (FPs), and dense particles (DPs), were obtained and characterized after purification. The immunogenicities of EP, FP, and the EP and FP mixture were evaluated in mice. Mouse-adapted CV-A5 was generated as a challenge strain to infect 14-day-old mice. An active immunization challenge mouse model was established to evaluate the efficacy of the inactivated vaccine candidate. This animal model mimics vaccination, similar to immune responses of the vaccinated. The animal model also tests protective efficacy in response to the vaccine against the disease. This work is important for the preparation of multivalent vaccines against HFMD caused by different emerging strains.
Keywords: CV-A5 vaccine; active immunization; efficacy; mouse model.
Copyright © 2021 American Society for Microbiology.
Figures



Similar articles
-
Efficacy of a coxsackievirus A6 vaccine candidate in an actively immunized mouse model.Emerg Microbes Infect. 2021 Dec;10(1):763-773. doi: 10.1080/22221751.2021.1906755. Emerg Microbes Infect. 2021. PMID: 33739899 Free PMC article.
-
Efficacy of Coxsackievirus A2 vaccine candidates correlating to humoral immunity in mice challenged with a mouse-adapted strain.Vaccine. 2022 Aug 5;40(33):4716-4725. doi: 10.1016/j.vaccine.2022.06.021. Epub 2022 Jun 25. Vaccine. 2022. PMID: 35760737
-
Immunological and biochemical characterizations of coxsackievirus A6 and A10 viral particles.Antiviral Res. 2016 May;129:58-66. doi: 10.1016/j.antiviral.2016.02.008. Epub 2016 Feb 17. Antiviral Res. 2016. PMID: 26899790
-
Production of EV71 vaccine candidates.Hum Vaccin Immunother. 2012 Dec 1;8(12):1775-83. doi: 10.4161/hv.21739. Epub 2012 Sep 19. Hum Vaccin Immunother. 2012. PMID: 22992566 Free PMC article. Review.
-
Recent development of enterovirus A vaccine candidates for the prevention of hand, foot, and mouth disease.Expert Rev Vaccines. 2018 Sep;17(9):819-831. doi: 10.1080/14760584.2018.1510326. Epub 2018 Aug 22. Expert Rev Vaccines. 2018. PMID: 30095317 Review.
Cited by
-
Viruses as tools in gene therapy, vaccine development, and cancer treatment.Arch Virol. 2022 Jun;167(6):1387-1404. doi: 10.1007/s00705-022-05432-8. Epub 2022 Apr 24. Arch Virol. 2022. PMID: 35462594 Free PMC article. Review.
-
A review of enterovirus-associated hand-foot and mouth disease: preventive strategies and the need for a global enterovirus surveillance network.Pathog Glob Health. 2024 Oct-Dec;118(7-8):538-548. doi: 10.1080/20477724.2024.2400424. Epub 2024 Sep 4. Pathog Glob Health. 2024. PMID: 39229797 Free PMC article. Review.
-
Identification of a Conserved, Linear Epitope on VP3 of Enterovirus A Species Recognized by a Broad-Spectrum Monoclonal Antibody.Viruses. 2023 Apr 21;15(4):1028. doi: 10.3390/v15041028. Viruses. 2023. PMID: 37113008 Free PMC article.
-
Emergence of a novel recombinant of CV-A5 in HFMD epidemics in Xiangyang, China.BMC Med Genomics. 2021 Nov 24;14(1):279. doi: 10.1186/s12920-021-01107-6. BMC Med Genomics. 2021. PMID: 34819054 Free PMC article.
-
A single amino acid mutation in VP1 of coxsackievirus A6 determining efficiency of VP0 cleavage and proliferation.J Virol. 2025 Jun 17;99(6):e0012825. doi: 10.1128/jvi.00128-25. Epub 2025 May 14. J Virol. 2025. PMID: 40366174 Free PMC article.
References
-
- Hu YF, Yang F, Du J, Dong J, Zhang T, Wu ZQ, Xue Y, Jin Q. 2011. Complete genome analysis of coxsackievirus A2, A4, A5, and A10 strains isolated from hand, foot, and mouth disease patients in China revealing frequent recombination of human enterovirus A. J Clin Microbiol 49:2426–2434. doi:10.1128/JCM.00007-11. - DOI - PMC - PubMed
-
- Zhang FK, Lu J, Wu J, Wang ZJ, Ji YQ, Zheng XL, Shen S. 2016. Immunogenicity of full and empty particles of coxsackievirus A16. Chin J Biol 28:1132–1137. doi:10.13200/j.cnki.cjb.001133. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

