Recommendations for SARS-CoV-2/COVID-19 testing: a scoping review of current guidance
- PMID: 33408209
- PMCID: PMC7789202
- DOI: 10.1136/bmjopen-2020-043004
Recommendations for SARS-CoV-2/COVID-19 testing: a scoping review of current guidance
Abstract
Background: Testing used in screening, diagnosis and follow-up of COVID-19 has been a subject of debate. Several organisations have developed formal advice about testing for COVID-19 to assist in the control of the disease. We collated, delineated and appraised current worldwide recommendations about the role and applications of tests to control SARS-CoV-2/COVID-19.
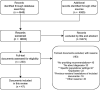
Methods: We searched for documents providing recommendations for COVID-19 testing in PubMed, EMBASE, LILACS, the Coronavirus Open Access Project living evidence database and relevant websites such as TRIP database, ECRI Guidelines Trust, the GIN database, from inception to 21 September 2020. Two reviewers applied the eligibility criteria to potentially relevant citations without language or geographical restrictions. We extracted data in duplicate, including assessment of methodological quality using the Appraisal of Guidelines for Research and Evaluation-II tool.
Results: We included 47 relevant documents and 327 recommendations about testing. Regarding the quality of the documents, we found that the domains with the lowest scores were 'Editorial independence' (Median=4%) and 'Applicability' (Median=6%). Only six documents obtained at least 50% score for the 'Rigour of development' domain. An important number of recommendations focused on the diagnosis of suspected cases (48%) and deisolation measures (11%). The most frequently recommended test was the reverse transcription-PCR (RT-PCR) assay (87 recommendations) and the chest CT (38 recommendations). There were 22 areas of agreement among guidance developers, including the use of RT-PCR for SARS-Cov-2 confirmation, the limited role of bronchoscopy, the use chest CT and chest X-rays for grading severity and the co-assessment for other respiratory pathogens.
Conclusion: This first scoping review of recommendations for COVID-19 testing showed many limitations in the methodological quality of included guidance documents that could affect the confidence of clinicians in their implementation. Future guidance documents should incorporate a minimum set of key methodological characteristics to enhance their applicability for decision making.
Keywords: diagnostic microbiology; epidemiology; protocols & guidelines.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; KSK has been paid for developing and delivering educational presentations for Ferring Pharmaceuticals and Olympus company; KSK has been an editor of medical Journals including BJOG, EBM-BMJ, BMC Med Edu; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- World Health Organization Coronavirus disease 2019 (COVID-19): situation report – 102. Switzerland: Geneve, 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
