SPC25 overexpression promotes tumor proliferation and is prognostic of poor survival in hepatocellular carcinoma
- PMID: 33408271
- PMCID: PMC7880370
- DOI: 10.18632/aging.202329
SPC25 overexpression promotes tumor proliferation and is prognostic of poor survival in hepatocellular carcinoma
Abstract
Background: The nuclear division cycle 80 (NDC80) complex assures proper chromosome segregation during the cell cycle progression. SPC25 is a crucial component of NDC80, and its role in hepatocellular carcinoma (HCC) has been explored recently. This study characterized the differential expression of SPC25 in HCC patients of different races and HBV infection status.
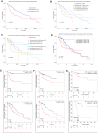
Methods: Expression patterns of SPC25 were evaluated in TCGA and Chinese HCC patients. Kaplan-Meier analysis was applied to examine the predictive value of SPC25. In vitro and in vivo functional assays were conducted to explore the role of SPC25 in HCC. Bioinformatics methods were applied to investigate the regulatory mechanisms of SPC25.
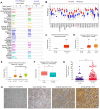
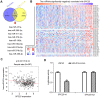
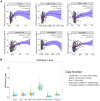
Findings: The mRNA levels of SPC25 were up-regulated in HCC. SPC25 has a significantly higher transcriptional level in Asian patients than Caucasian patients. SPC25 promoted HCC cell proliferation in vitro and tumor growth in vivo by accelerating the cell cycle. We identified transcription factors, miRNAs, and immune cells that may interact with SPC25.
Interpretation: The findings suggest that increased expression of SPC25 is associated with poor prognosis of HCC and enhances the proliferative capacity of HCC cells. SPC25 could serve as a valuable prognostic marker and a novel treatment target for HCC.
Keywords: HCC prognosis; HCC progression; SPC25.
Conflict of interest statement
Figures




References
-
- Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle AS, Abebe ND, Abraha HN, Abu-Raddad LJ, et al., and Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019; 5:1749–68. 10.1001/jamaoncol.2019.2996 - DOI - PMC - PubMed
-
- Janke C, Ortiz J, Lechner J, Shevchenko A, Shevchenko A, Magiera MM, Schramm C, Schiebel E. The budding yeast proteins Spc24p and Spc25p interact with Ndc80p and Nuf2p at the kinetochore and are important for kinetochore clustering and checkpoint control. EMBO J. 2001; 20:777–91. 10.1093/emboj/20.4.777 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

