Randomized Phase II Study to Comparing Docetaxel/Nedaplatin versus Docetaxel for 5-Fluorouracil/Cisplatin Resistant Esophageal Squamous Cell Carcinoma
- PMID: 33408308
- PMCID: PMC8374090
- DOI: 10.5761/atcs.oa.20-00294
Randomized Phase II Study to Comparing Docetaxel/Nedaplatin versus Docetaxel for 5-Fluorouracil/Cisplatin Resistant Esophageal Squamous Cell Carcinoma
Abstract
Purpose: To compare efficacy and safety of dual docetaxel/nedaplatin treatment versus docetaxel alone as second-line chemotherapy for advanced esophageal cancer.
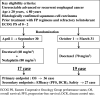
Methods: In all, 36 patients with metastatic and/or recurrent esophagus squamous cell carcinoma resistant to first-line chemotherapy (fluorouracil/cisplatin) were recruited from 2011 to 2018 and randomized into two groups. Treatment response and survival were compared between the docetaxel/nedaplatin (60/80 mg/m2/day) group and docetaxel (70 mg/m2/day) group. Treatment was repeated every 3 weeks until tumor progression. Patients were followed up until March 2019 or death.
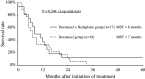
Results: The frequency of Grade 3 or higher adverse events in the docetaxel/nedaplatin group (58.8%) was higher compared with the docetaxel group (26.3%) (P = 0.090). We found a treatment response rate of 52.9% and 36.8% and a median survival of 8.9 and 7.0 months in the docetaxel/nedaplatin-treated and docetaxel-treated group, respectively (P = 0.544).
Conclusion: No significant survival advantage was found for docetaxel/nedaplatin-treated patients, although there was an increased frequency of high-grade adverse events compared to docetaxel-treated patients. Because of the limited cohort size, a Phase III study based on our findings is not warranted to assess the clinical impact of docetaxel/nedaplatin treatment. This trial is registered with the University Hospital Medical Information Network (UMIN 000005877).
Keywords: Phase II study; docetaxel; esophageal squamous cell carcinoma; nedaplatin; second-line treatment.
Figures
Similar articles
-
Neoadjuvant chemotherapy with docetaxel, nedaplatin, and fluorouracil for resectable esophageal cancer: A phase II study.Cancer Sci. 2018 Nov;109(11):3554-3563. doi: 10.1111/cas.13772. Epub 2018 Sep 25. Cancer Sci. 2018. PMID: 30137686 Free PMC article. Clinical Trial.
-
Phase I Study of Docetaxel Plus Nedaplatin in Patients With Metastatic or Recurrent Esophageal Squamous Cell Carcinoma After Cisplatin Plus 5-Fluorouracil Treatment.Am J Clin Oncol. 2016 Feb;39(1):13-7. doi: 10.1097/COC.0000000000000018. Am J Clin Oncol. 2016. PMID: 24322336 Clinical Trial.
-
Phase II Trial of 5-Fluorouracil, Docetaxel, and Nedaplatin (UDON) Combination Therapy for Recurrent or Metastatic Esophageal Cancer.Oncologist. 2019 Feb;24(2):163-e76. doi: 10.1634/theoncologist.2018-0653. Epub 2018 Oct 25. Oncologist. 2019. PMID: 30361422 Free PMC article. Clinical Trial.
-
Phase II trial of neoadjuvant chemotherapy with docetaxel, nedaplatin, and S1 for advanced esophageal squamous cell carcinoma.Cancer Sci. 2016 Jun;107(6):764-72. doi: 10.1111/cas.12943. Epub 2016 May 12. Cancer Sci. 2016. PMID: 27061001 Free PMC article. Clinical Trial.
-
Effectiveness of taxanes following nivolumab in patients with advanced esophageal squamous cell carcinoma: a retrospective chart review of patients in ATTRACTION-3.Esophagus. 2023 Apr;20(2):302-308. doi: 10.1007/s10388-022-00972-z. Epub 2022 Dec 23. Esophagus. 2023. PMID: 36564602 Free PMC article. Review.
Cited by
-
Sugemalimab combined with chemotherapy for the treatment of advanced esophageal squamous cell carcinoma: a cost-effectiveness analysis.Front Pharmacol. 2024 Jun 28;15:1396761. doi: 10.3389/fphar.2024.1396761. eCollection 2024. Front Pharmacol. 2024. PMID: 39005941 Free PMC article.
-
Preoperative Low Serum Calcium Levels Predict Poor Prognosis for Patients with Esophageal Cancer.Ann Thorac Cardiovasc Surg. 2022 Apr 20;28(2):96-102. doi: 10.5761/atcs.oa.21-00167. Epub 2021 Sep 23. Ann Thorac Cardiovasc Surg. 2022. PMID: 34556614 Free PMC article.
-
Combination of high anti-SKI and low anti-TMED5 antibody levels is preferable prognostic factor in esophageal carcinoma.Cancer Sci. 2024 Jul;115(7):2209-2219. doi: 10.1111/cas.16185. Epub 2024 Apr 18. Cancer Sci. 2024. PMID: 38634426 Free PMC article.
-
Novel cancer-specific epidermal growth factor receptor antibody obtained from the serum of esophageal cancer patients with long-term survival.Cancer Sci. 2022 Jun;113(6):2118-2128. doi: 10.1111/cas.15350. Epub 2022 Apr 18. Cancer Sci. 2022. PMID: 35348270 Free PMC article.
References
-
- Cancer statistics in Japan, Cancer Information Service. Center of cancer control and information services NCC. [cited 2018 Apr 10]. Available from:. Available from https:// ganjoho.jp/en/professional/statistics/table_download.html.

