Activation and disruption of a neural mechanism for novel choice in monkeys
- PMID: 33408410
- PMCID: PMC7116896
- DOI: 10.1038/s41586-020-03115-5
Activation and disruption of a neural mechanism for novel choice in monkeys
Abstract
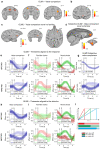
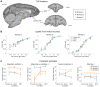
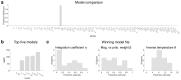
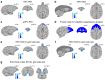
Neural mechanisms that mediate the ability to make value-guided decisions have received substantial attention in humans and animals1-6. Experiments in animals typically involve long training periods. By contrast, choices in the real world often need to be made between new options spontaneously. It is therefore possible that the neural mechanisms targeted in animal studies differ from those required for new decisions, which are typical of human imaging studies. Here we show that the primate medial frontal cortex (MFC)7 is involved in making new inferential choices when the options have not been previously experienced. Macaques spontaneously inferred the values of new options via similarities with the component parts of previously encountered options. Functional magnetic resonance imaging (fMRI) suggested that this ability was mediated by the MFC, which is rarely investigated in monkeys3; MFC activity reflected different processes of comparison for unfamiliar and familiar options. Multidimensional representations of options in the MFC used a coding scheme resembling that of grid cells, which is well known in spatial navigation8,9, to integrate dimensions in this non-physical space10 during novel decision-making. By contrast, the orbitofrontal cortex held specific object-based value representations1,11. In addition, minimally invasive ultrasonic disruption12 of MFC, but not adjacent tissue, altered the estimation of novel choice values.
Conflict of interest statement
Figures








References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

