Control of osteoblast regeneration by a train of Erk activity waves
- PMID: 33408418
- PMCID: PMC7864885
- DOI: 10.1038/s41586-020-03085-8
Control of osteoblast regeneration by a train of Erk activity waves
Abstract
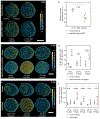
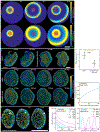
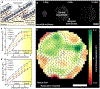
Regeneration is a complex chain of events that restores a tissue to its original size and shape. The tissue-wide coordination of cellular dynamics that is needed for proper morphogenesis is challenged by the large dimensions of regenerating body parts. Feedback mechanisms in biochemical pathways can provide effective communication across great distances1-5, but how they might regulate growth during tissue regeneration is unresolved6,7. Here we report that rhythmic travelling waves of Erk activity control the growth of bone in time and space in regenerating zebrafish scales, millimetre-sized discs of protective body armour. We find that waves of Erk activity travel across the osteoblast population as expanding concentric rings that are broadcast from a central source, inducing ring-like patterns of tissue growth. Using a combination of theoretical and experimental analyses, we show that Erk activity propagates as excitable trigger waves that are able to traverse the entire scale in approximately two days and that the frequency of wave generation controls the rate of scale regeneration. Furthermore, the periodic induction of synchronous, tissue-wide activation of Erk in place of travelling waves impairs tissue growth, which indicates that wave-distributed Erk activation is key to regeneration. Our findings reveal trigger waves as a regulatory strategy to coordinate cell behaviour and instruct tissue form during regeneration.
Figures











References
Additional References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

