Impact of Programmed Death Ligand 1 Expression in Advanced Non-Small-Cell Lung Cancer Patients, Treated by Chemotherapy (GFPC 06-2015 Study)
- PMID: 33408480
- PMCID: PMC7779294
- DOI: 10.2147/OTT.S288825
Impact of Programmed Death Ligand 1 Expression in Advanced Non-Small-Cell Lung Cancer Patients, Treated by Chemotherapy (GFPC 06-2015 Study)
Abstract
Background: Few data have been published on the clinical and histopathological characteristics of advanced non-small-cell lung cancer (NSCLC) patients with high PD-L1 expression versus intermediate or none and the prognostic value of PD-L1 expression for patients treated with chemotherapy is unknown. This study was undertaken to prospectively assess the prognostic value of tumor-cell (TC) and immune-cell (IC) PD-L1 expressions for advanced NSCLC patients.
Methods: It was a prospective, multicenter study on advanced NSCLC patients, with performance status 0/1, scheduled, consecutively, to receive first-line platin-based chemotherapy. PD-L1 expression was determined immunochemically (Dako Autostainer and monoclonal antibody 22C3) and its impact on progression-free survival (PFS) and overall survival (OS) assessed.
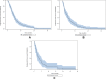
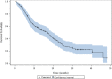
Results: Among 198 patients screened in 19 centers, 140 were included median age: 66.5 ± 10 years; 76.4% men; 79.3% Caucasians; 10.7% nonsmokers; 63.6% adenocarcinomas; <1%, 1-50% and ≥50% TC PD-L1-expression rates were 47.1%, 25.7% and 27.2% of patients, respectively; respective null, intermediate and high rates on ICs were 35.7%, 38.6% and 25.7%. Second- and third-line chemotherapies were administered to 58.6% and 26.4% of the patients, respectively. None received immunotherapy. First-, second- and third-line median (95% CI) PFS lasted 4.6 (3.6-5.2), 3.7 (2.3-4.7) and 2.2 (1.5-4.3) months, respectively; median OS was 16.9 (11.4-19.9) months. No significant PFS and OS differences were observed according to TC or IC PD-L1 expression.
Conclusion: According to the results of this prospective, multicenter study, neither TC nor IC PD-L1 expression appears to be prognostic for chemotherapy-managed advanced NSCLC patients.
Keywords: PD-L1; chemotherapy; immunotherapy; non-small–cell lung cancer; prognostic.
© 2020 Auliac et al.
Conflict of interest statement
Dr Jean-Bernard Auliac reports grants, personal fees, non-financial support from Astra Zeneca and MSD; grants from BMS and Roche; personal fees from Amgen and Boehringer Ingelheim, during the conduct of the study. Dr Florian Guisier reports personal fees, non-financial support from BMS, MSD, Roche, Boehringer Ingelheim, and Astra Zeneca, outside the submitted work. Professor Christos Chouaïd reports grants, personal fees, non-financial support from Roche, MSD, AZ, BMS, Amgen, Lilly, BI, GSK, Janssen, Pfizer, Takeda, and Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials